Summary.
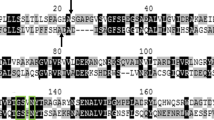
The Na+, K+-ATPase of the Monarch butterfly (Danaus plexippus) is insensitive to the inhibition by cardiac glycosides due to an amino acid replacement: histidine instead of asparagine at position 122 of the α-subunit representing the ouabain binding site. By PCR amplification of the DNA sequence of this site, a PCR product of 270 bp was obtained from DNA extracted from Danainae species (Danaus plexippus, D. chrysippus, D. gillipus, D. philene, D. genutia, Tirumala hamata, Euploea spp., Parantica weiskei, P. melusine), Sphingidae (Daphnis nerii) and mimics of milkweed butterflies (Hypolimnas missipus, Limenitis archippus and L. arthemis, Nymphalidae). Analysis of the nucleotide sequences revealed that the single point mutation in the ouabain binding domain (AAC-Asn for CAC-His) was present only in Danaus plexippus, but not in the other species investigated. Since these milkweed butterflies also store cardenolides, other structural modifications of the Na+, K+-ATPase may have occurred or other strategies of cardenolide tolerance have been developed.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 15 May 2000; accepted 29 June 2000
Rights and permissions
About this article
Cite this article
Mebs, D., Zehner, R. & Schneider, M. Molecular studies on the ouabain binding site of the Na+, K+-ATPase in milkweed butterflies. Chemoecology 10, 201–203 (2000). https://doi.org/10.1007/PL00001823
Issue Date:
DOI: https://doi.org/10.1007/PL00001823