Abstract
Background: The SF-36 is widely used to assess health-related quality of life (HRQOL), but with few longitudinal studies in healthy populations, it is difficult to quantify its natural history. This is important because any measure of change following an intervention may be confounded by natural changes in HRQOL. This paper assesses mean changes in SF-36 scores over a 3-year period in men and women between the ages of 40 and 59 years at baseline.
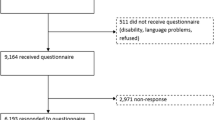
Methods: Subjects were randomly selected from nine Canadian cities. Mean SF-36 changes over a 3-year period (1996/1997-1999/2000) were calculated for each gender within 5-year age categories. Multiple imputation was used to correct for potential bias due to missing data.
Results: The baseline cohort included 1,974 women and 975 men between 40 and 59 years. Mean changes in HRQOL tended to be small. Women demonstrated small average declines in 22 of the 32 age and domain groupings (4 age groups, 8 SF-36 domains) while men showed declines in 14/32. Most participants stayed within 10 points of their original baseline score.
Interpretation: Mean SF-36 scores change only slightly over three years in middle-aged Canadians, although there is much individual variation. It may be necessary to adjust for the natural evolution of SF-36 scores when interpreting results from longitudinal studies.
Résumé
Contexte: Le SF-36 est un court questionnaire très utilisé pour évaluer la qualité de vie liée à la santé (HRQOL). Toutefois, vu le petit nombre d’études longitudinales menées auprès de populations en bonne santé, il est difficile d’évaluer l’histoire naturelle du SF-36, ce qui est important parce que tout changement noté résultant d’une intervention peut être confondu avec des changements normaux de la qualité de vie liée à la santé. Cet article évalue les changements moyens dans les scores des SF-36 sur une période de trois ans chez les hommes et les femmes qui étaient âgés de 40 à 59 ans lors de leur visite initiale.
Méthode: Les sujets ont été choisis au hasard dans neuf villes canadiennes. Les changements moyens dans les résultats du SF-36 sur une période de trois ans (1996–1997 à 1999–2000) ont été calculés par sexe et par groupe d’âge de cinq ans. L’imputation multiple a été utilisée pour corriger tout biais résultant d’informations manquantes.
Résultats: La cohorte initiale comportait 1 974 femmes et 975 hommes âgés de 40 à 59 ans. Les changements moyens étaient plutôt légers. Chez les femmes, 22 des 32 groupes d’âge et sous-échelles (quatre groupes d’âge, huit sous-échelles SF-36) présentaient de légères diminutions moyennes, alors que chez les hommes, ce nombre était de 14 groupes sur 32. Le score de la plupart des participants est demeuré en deçà de 10 points du score initial lors de l’entrée dans l’étude.
Interprétation: Les scores moyens du SF-36 ne changent que légèrement sur une période de trois ans dans la population canadienne d’âge moyen. Toutefois, il pourrait s’avérer nécessaire de tenir compte de l’évolution naturelle des scores du SF-36 lors de l’interprétation des résultats d’études longitudinales.
Similar content being viewed by others

Reference
Ware JE Jr, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. The Health Institute, New England Medical Center, Boston, 1993.
Ware JE Jr, Kosinski M, Keller SD. SF-36 Physical and Mental Summary Scales: A User’s Manual. The Health Institute, New England Medical Center, Boston, 1994.
Ware JE Jr. SF-36 health survey updat. Spin. 2000;25:3130–39.
Kaegi L. Medical Outcomes Trust Conference presents dramatic advances in patient-based outcomes assessment and potential applications in accreditation. Joint Commission Journal of Quality Improvement. 1999;25:207–18.
Garratt A, Schmidt L, Mackintosh A, Fitzpatrick R. Quality of life measurement: Bibliographic study of patient assessed health outcomes measure. Br Med. 2002;324:1417–21.
Hemingway H, Stafford M, Stansfield S, Shipley M, Marmot M. Is the SF-36 a valid measure of change in population health? Results from the Whitehall II study. Br Med. 1997;315:1273–79.
Hopman WM, Towheed T, Anastassiades T, Tenenhouse A, Poliquin S, Berger C, et al. and the CaMos Research Group. Canadian Normative Data for the SF-36 Health survey. CMA. 2000;163:265–71.
Chern JY, Wan TT, Pyles M. The stability of health status measurement (SF-36) in a working populatio. J Outcome Measure. 2000;4:461–81.
Hays RD, Marshall GN, Wang EY, Sherbourne CD. Four-year cross-lagged associations between physical and mental health in the Medical Outcomes Stud. J Consulting Clinical Psycholog. 1994;62:441–49.
Hopman WM, Towheed T, Anastassiades T, Tenenhouse A, Poliquin S, Berger C, et al. and the CaMos Research Group. Is there regional variation in the SF-36 scores of Canadian adult. Can J Public Healt. 2002;93:233–36.
Guyatt G, Walter S, Norman G. Measuring change over time: Assessing the usefulness of evaluative instrument. J Chron Dis. 1987;40:171–78.
Testa MA. Interpretation of quality-of-life outcomes: Issues that affect magnitude and meaning. Med Care. 2000;38(Supplement II):166–74.
Liang MH. Longitudinal construct validity: Establishment of clinical meaning in patient evaluative instrument. Med Car. 2000;38(Supplement II):84–90.
Kreiger N, Tenenhouse A, Joseph L, MacKenzie T, Poliquin S, Brown J, et al. Research Notes: The Canadian Multicentre Osteoporosis Study (CaMos): Background, Rationale, Method. Can J Aging. 1999;18:376–87.
Rubin D. Multiple Imputation for Non-response in Surveys. New York: Wiley, 1987.
Kass RE, Raftery AE. Bayes factor. J Am Statistical Association. 1995;90:773–95.
Author information
Authors and Affiliations
Corresponding author
Additional information
See Acknowledgements for Complete List
Acknowledgements: CaMos was funded by the Senior’s Independence Research Program through the National Health Research and Development Program (project no. 6605-4003-OS), the Medical Research Council of Canada-Pharmaceutical Manufacturers Association of Canada (MRC-PMAC) Health Program, Merck Frosst Canada Inc., Eli Lilly Canada Inc., Procter & Gamble Pharmaceuticals Canada, Inc., the Dairy Farmers of Canada, The Arthritis Society.
Rights and permissions
About this article
Cite this article
Hopman, W.M., Berger, C., Joseph, L. et al. Stability of Normative Data for the SF-36. Can J Public Health 95, 387–391 (2004). https://doi.org/10.1007/BF03405153
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03405153