Abstract
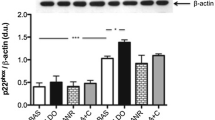
Clinical studies have demonstrated that aldosterone receptor antagonists do improve the survival of patients with chronic heart diseases and in vitro studies have shown that canrenone blocks the proinflammatory effect of aldosterone in mononucler leukocytes (MNL). The aim of the study was to compare, in the model of human MNL, the effect of potassium-sparing diuretics amiloride and canrenone, on the protein expression of p22phox, a NADPH-oxidase system subunit, that is a principal marker of production of superoxide anions. MNL were isolated from 10 informed healthy volunteers (5 males and 5 females, age range 24–36 yr) and the proteins extracted. p22phox protein expression was evaluated by Western blot and quantified using a densitometric semiquantitative analysis. The experiments showed that aldosterone (10−8 M) enhances the protein expression of p22phox and that its effect is reversed by co-incubation with canrenone (10−6 M), while incubation with amiloride (10−6 M) reduced the prooxidative effect of aldosterone at a significantly lower extent than canrenone. Co-incubation with canrenone, amiloride, and aldosterone together produced the same effect as aldosterone plus canrenone. Incubation with cortisol (40−8 M) was not effective. These data confirm the prooxidative effect of aldosterone in MNL. The addition of aldosterone-receptor antagonist canrenone produced a higher inhibition than sodium channel blocker amiloride on the effect of aldosterone on p22phox protein expression.
Similar content being viewed by others
References
Rocha R, Martin-Berger CL, Yang P, Scherrer R, Delyani J, McMahon E. Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology 2002, 143: 4828–36.
Karram T, Abbasi A, Keidar S, et al. Effects of spironolactone and eposartan on cardiac remodelling and angiotensin-converting enzyme isoforms in rats with experimental heart failure. Am J Physiol Heart Circ Physiol 2005, 289: H1351–8.
Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999, 341: 709–17.
Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone a selective aldosterone blocker in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003, 348: 1309–21.
Weber KT, Anversa P, Armstrong PW, et al. Remodeling and reparation of the cardiovascular system. J Am Coll Cardiol 1992, 20: 3–16.
Yasunari K, Maeda K, Nakamura M, Yoshikawa J. Oxidative stress in leukocytes is a possible link between blood pressure, blood glucose, and C-reacting protein. Hypertension 2002, 39: 777–80.
Gerling IC, Sun Y, Ahokas RA, et al. Aldosteronism: an immunostimulatory state precedes proinflammatory/fibrogenic cardiac phenotype. Am J Physiol Heart Circ Physiol 2003, 285: H813–21.
Mervaala EM, Muller DN, Park JK, et al. Monocyte infiltration and adhesion molecules in a rat model of high renin hypertension. Hypertension 1999, 33: 389–95.
Sun Y, Zhang J, Lu L, et al. Aldosterone-induced inflammation in the rat heart. Role of oxidative stress. Am J Pathol 2002, 161: 1773–81.
Keidar S, Hayek T, Kaplan M, et al. Effect of eplerenone, a selective aldosterone blocker, on blood pressure, serum and macrophage oxidative stress, and arteriosclerosis in apolipoprotein-E deficient mice. J Cardiovasc Pharmacol 2003, 41: 955–63.
Keidar S, Kaplan M, Pavlotzky E, et al. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation 2004, 109: 2213–20.
Takai S, Jin D, Muramatsu M, et al. Eplerenone inhibits atherosclerosis in nonhuman primates. Hypertension 2005, 46: 1135–9.
Park YM, Park MY, Suh YL, Park JB. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone-infused rats. Biochem Biophys Res Commun 2004, 313: 812–7.
Armanini D, Strasser T, Weber PC. Characterization of aldosterone binding sites in circulating human mononuclear leucocytes. Am J Physiol 1985, 248: E388–90.
Wehling M, Armanini D, Strasser T, Weber PC. Effect of aldosterone on sodium and potassium concentration in human mononuclear leucocytes. Am J Physiol 1987, 252: E505–8.
Wehling M, Kuhls S, Armanini D. Volume regulation of human lymphocytes by aldosterone in isotonic media. Am J Physiol 1989, 20: E170–4.
Calò LA, Zaghetto F, Pagnin E, et al. Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22(phox) in human mononuclear leukocytes. J Clin Endocrinol Metab 2004, 89: 1973–6.
Haddad JJ. Amiloride and the regulation of NF-κB: an unsung crosstalk and missing link between fluid dynamics and oxidative stress-related inflammation-controversy or pseudocontroversy? Biochem Biophys Res Commun 2005, 327: 373–81.
Garciandia A, López R, Tisaire J, et al. Enhanced Na(+)−H+ exchanger activity and NHE-1 mRNA expression in lymphocytes from patients with essential hypertension. Hypertension 1995, 25: 356–64.
De Vito P. The sodium/hydrogen exchanger: a possible mediator of immunity. Cell Immunol 2006, 240: 69–85.
Bubien JK, Warnock DG. Amiloride-sensitive sodium conductance in human B lymphoid cells. Am J Physiol 1993, 265: C1175–83.
Ottaviani E, Franchini A, Mandrioli M, Saxena A, Hanukoglu A, Hanukoglu I. Amiloride-sensitive epithelial sodium channel sub-units are expressed in human mussel immunocytes. Dev Comp Immunol 2002, 26: 395–402.
Bellocq A, Suberville S, Philippe C, et al. Low environmental pH is responsible for the induction of nitric-oxide synthase in macrophages. J Biol Chem 1998, 273: 5086–92.
Friedman M. The use of ranks to avoid the assumption of normality implicit in the analysis of variance. J Am Stat Assoc 1937, 32: 675–701.
Wilcoxon F. Individual comparisons by ranking methods. Biometrics 1945, 1: 80–3.
Michea L, Delpiano AM, Hitschfeld C, Lobos L, Lavandero S, Marusic ET. Eplerenone blocks nongenomic effects of aldosterone on the Na+/H+ exchanger, intracellular Ca2+ levels, and vasoconstriction in mesenteric resistance vessels. Endocrinology 146, 3: 973–80.
Fiebeler A, Luft FC. The mineralocorticoid receptor and oxidative stress. Heart Fail Rev 2005, 10: 47–52.
Piwien-Pilipuk G, Ayala A, Machado A, Galigniana D. Impairment of mineralocorticoid receptor (MR)-dependent biological response by oxidative stress and aging: correlation with post-translational modification of MR and decreased ADP-ribosylatable level of elongation factor 2 in kidney cells. J Biol Chem 2002, 277: 11896–903.
Calò LA, Armanini D. Aldosterone and thrombosis formation: implications for ischemic and atherosclerotic heart disease. J Endocrinol Invest 2006, 29: 675–6.
Armanini D, Scaroni C, Mattarello MJ, Fiore C, Albiger N, Sartorato P. Idiopathic primary hyperaldosteronism: normalization of plasma aldosterone after one month withdrawal of long-term therapy with aldosterone-receptor antagonist potassium canrenoate. J Endocrinol Invest 2005, 28: 236–40.
Ma J, Albornoz F, Yu C, Byrne DW, Vaughan DE, Brown NJ. Differing effects of mineralocorticoid receptor-dependent and -independent potassium-sparing diuretics on fibrinolitic balance. Hypertension 2005, 46: 313–20.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fiore, C., Sartorato, P., Pagnin, E. et al. Effect of canrenone and amiloride on the prooxidative effect induced by aldosterone in human mononuclear leukocytes in vitro . J Endocrinol Invest 32, 895–898 (2009). https://doi.org/10.1007/BF03345768
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03345768