Abstract
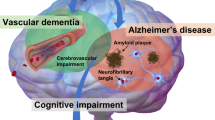
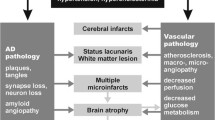
The hypothesis that vascular factors may contribute to the development of Alzheimer’s disease (AD) is supported by epidemiologic and pathologic observations. Arterial hypertension and diabetes have been found to be associated not only with vascular dementia, but also with AD; in addition, the treatment of hypertension with calcium antagonists seems to prevent degenerative dementias. Hypertension and hyperinsulinemia favor the deposition of amyloid substance in the brain. The histopathology of AD is marked not only by neurofibrillary tangles and senile plaques, but also by macro and micro congophilic angiopathy and ischemic white matter rarefaction. The specific AD pathological lesions, if isolated, are not able to lead to an evident clinical picture of dementia, which, on the contrary, becomes evident when vascular, mainly subcortical, lesions are associated. These and other observations suggest that vascular factors may have a role in the development of AD. An aggressive approach to these factors could be of value in the prevention of AD.
Similar content being viewed by others
References
American Psychiatric Association: Diagnostic and statistical manual of mental disorders, 4th ed. revised. Washington, DC, APA, 1996.
McKhann G., Drachman D., Folstein M.: Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA. Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34: 939–944, 1984.
White L.: Is silent cerebrovascular disease an important cause of late-life cognitive decline? J. Am. Geriatr. Soc. 44: 328–330, 1996.
Elias M.F.: Effects of chronic hypertension on cognitive functioning. Geriatrics 53 (Suppl. 1): S49–S52, 1998.
Elias M.F., Wolf P.A., D’Agostino R.B.: Untreated blood pressure level is inversely related to cognitive functioning. The Framingham Study. Am. J. Epidemiol. 138: 353–364, 1993.
Kilander L., Nyman H., Boberg M., Hansson L., Lithell H.: Hypertension is related to cognitive impairment. A 20-years follow-up of 999 men. Hypertension 31: 780–786, 1998.
Launer L.J., Masaki K., Petrovitch H.: The association between midlife blood pressure levels and lateLife cognitive function. JAMA 274: 1846–1851, 1995.
Palombo V., Scurti R., Muscari A., Puddu G.M., Di Iorio A., Zito M., Abate G.: Blood pressure and intellectual function in elderly subjects. Age Ageing 26: 91–98, 1997.
Skoog I., Lernfelt B., Landahl S.: 15-year longitudinal study of blood pressure and dementia. Lancet 349: 151–154, 1997.
Skoog I., Landahl S., Lernfelt B.: High blood pressure and dementia. Lancet 348: 65–66, 1996 (letter).
Skoog I., Andreasson L., Landhal S., Lernfelt B.: A population-based study on blood pressure and brain atrophy in 85-years olds. Hypertension 32: 404–409, 1998.
Guo Z., Vitanen M., Fratiglioni L.: Low blood pressure and dementia in elderly people: the Kungsholmen project. BMJ 312: 805–808, 1996.
Leibson C.L., Rocca W.A., Hanson V.A.: Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am. J. Epidemiol. 145: 301–308, 1997.
Yoshitake T., Kiyohara Y., Kato I.: Incidence and risk factors of vascular dementia and Alzheimer’s disease in defined elderly Japanese population. The Hisayama Study. Neurology 45: 1161–1168, 1995.
Yan S.D., Chen X., Fu J.: RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease patients. Stroke 27: 2069–2074, 1996.
Mooradian A.D.: Blood-brain barrier choline transport is reduced in diabetic rats. Diabetes 36: 1094–1097, 1987.
Kuusisto J., Koivisto K., Mykkanen L., Helkala E.L., Vanhanen M., Hanninen T., Pyorala K., Rikkinen P., Laakso M.: Essential hypertension and cognitive function. The role of hyperinsulinemia. Hypertension 22: 771–779, 1993.
Kuusisto J., Koivisto K., Mykkanen L.: Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: Cross sectional population based study. BMJ 315: 1045–1049, 1997.
Palovcik R.A., Phillips M.I., Kappy M.S.: Insulin inhibits pyramidal neurons in hippocampal slices. Brain Res. 309: 187–191, 1984.
Brass B.J., Nonner D., Barret J.N.: Differential effects of insulin on choline acetyltransferase and glutamic acid decarboxilase activities in neuron-rich striatal cultures. J. Neurochem. 59: 415–424, 1992.
Hong M., Lee V.M.Y.: Insulin and insulin-like growth factor-1 regulate tau phoshorylation in cultured human neurons. J. Biol. Chem. 272: 1947–1953, 1997.
Unger J., McNeill T.H., Moxley R.T.: Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience 31: 143–147, 1989.
Landin K., Blennow K., Wallin A.: Low blood pressure and blood glucose levels in Alzheimer’s disease. Evidence for a hypometabolic disorder? J. Intern. Med. 233: 357–363, 1993.
Wolf-Klein G.P., Silverstone F.A., Brod M.S.: Are Alzheimer patients healthier? J. Am. Geriatr. Soc. 36: 219–224, 1988.
Hofman A., Ott A., Breteler M.M.B.: Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet 349: 151–154, 1997.
Scott R.D., Krits-Silverstein D., Barrett-Connor E., Wiederholt W.C.: The association of non-insulin-dependent diabetes mellitus and cognitive function in an older cohort. J. Am. Geriatr. Soc. 46: 1217–1222, 1998.
Graves A.B., Van Duijn C.M., Chandra V.: Alcohol and tobacco consumption as risk factors for Alzheimer’s disease: a collaborative re-analysis of case-control studies. Int. J. Epidemiol. 20 (Suppl. 2): S48–S57, 1991.
Prince M., Cullen M., Mann A.: Risk factors for Alzheimer’s disease and dementia: a case-control study based on the MRC elderly hypertension trial. Neurology 44: 97–104, 1994.
Brenner D.E., Kukull W.A., Van Belle G., Bowen J.D., Mc-Cormick W.C., Teri L., Larson E.B.: Postmenopausal estrogen replacement therapy and the risk of Alzheimer’s disease: a population-based case-control study. Am. J. Epidemiol. 140: 262–267, 1994.
Whitehouse P.J., Kalaria R.N.: Nicotine receptors and neu-rodegenerative dementing diseases: basic research and clinical implications. Alzheimer Dis. Assoc. Disord. 9 (Suppl. 2): 3–5, 1995.
Wilson A.L., Langley L.K., Monley J., Bauer T., Rottunda S., McFalls E., Kovera C., McCarten J.R.: Nicotine patches in Alzheimer’s disease: pilot study on learning, memory and safety. Pharmacol. Biochem. Behav. 51: 509–514, 1995.
Canadian Study of Health and Aging Working Group. The Canadian Study of Health and Aging: risk factors for Alzheimer’s disease in Canada. Neurology 44: 2073–2080, 1994.
Forster D.P., Newens A.J., Kay D.W., Edwardson J.A.: Risk factors in clinically diagnosed presenile dementia of the Alzheimer type: a case-control study in northern England. J. Epidemiol. Community Health 49: 253–258, 1995.
Fielding J.E.: Smoking: health effect and control. N. Engl. J. Med. 313: 555–561, 1985.
Ferrucci L., Guralnik J.M., Salive M.E.: Cognitive impairment and risk of stroke in the older population. J. Am. Geriatr. Soc. 44: 237–241, 1996.
Moroney J.T., Desmond D.W: Cognitive impairment and risk of stroke in the older population. J. Am. Geriatr. Soc. 44: 1410–1411, 1996 (letter).
Ott A., Breteler M.M.B., de Bruyne M.C.: Atrial fibrillation and dementia in a population based study. Stroke 28: 316–321, 1997.
Kalman J., Juhasz A., Csaszar A., Kanka A., Rimanoczy A., Janka Z., Rasko I.: Increased apolipoprotein E4 allele frequency is associated with vascular dementia in the Hungarian population. Acta Neurol. Scand. 98: 166–168, 1998.
Noguchi S., Murakami K., Yamada N.: Apolipoprotein E genotype and Alzheimer’s disease. Lancet 342: 737–741, 1993.
Nagy Z.S., Esiri M.M., Joachim C., Jobst K.A., Morris J.H., King E.M., Hindley N.J., McDonald B., Litchfield S., Barnetson L., Smith A.D.: Comparison of pathological diagnostic criteria for Alzheimer disease. Alzheimer Dis. Assoc. Disord. 12: 182–189, 1998.
Morris J.C., Storandt M., McKeel D.W., Rubin E.H., Price J.L., Grant E.A., Berg L.: Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for presympto-matic and very mild Alzheimer’s disease. Neurology 46: 707–719, 1996.
Haroutunian V., Perl D.P., Purohit D.P., Marin D., Khan K., Lantz M., Davis K.L., Mohs R.C.: Regional distribution of neuritic plaques in the non demented elderly and subjects with very mild Alzheimer disease. Arch. Neurol. 55: 1185–1191, 1998.
Ellis R.J., Olichney J.M., Thal L.J., Mirra S.S., Morris J.C., Beekly D., Heyman A.: Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience. Part XV. Neurology 46: 1592–1596, 1996.
Kalaria R.N., Hedera P.: β-amyloid vasoactivity in Alzheimer’s disease. Lancet 347: 1492–1493, 1996 (letter).
de la Torre J.C., Mussivand T.: Can disturbed brain microcir-culation cause Alzheimer’s disease? Neurol. Res. 15: 146–153, 1993.
Sutton E.T., Hallermann G.R., Thomas T.: β-Amyloid induced endothelial necrosis and inhibition of nitric oxide production. Exp. Cell Res. 230: 368–376, 1997.
Olichney Y.M., Hansen L.A., Hofstetter C.R.: Cerebral infarction in Alzheimer’s disease is associated with severe amyloid angiopathy and hypertension. Arch. Neurol. 52: 703–708, 1995.
Iwamoto N., Nishiyama E., Ohwada J.: Distribution of amyloid deposits in the cerebral white matter of the Alzheimer’s disease brain: relationship to blood vessels. Acta Neu-ropathol. 93: 334–340, 1997.
Brillian M., Hughes L., Anderson D., Ghobrial M., Elble R.: Rarefied white matter in patients with Alzheimer disease. Alzheimer Dis. Assoc. Disord. 9: 39–46, 1995.
Golomb J., Kluger A., Gianutsos J., Ferris S.H., de Leon M.J., George A.E.: Non specific leukoencephalopathy associated with aging. Neuroimaging Clin. J. Am. 5: 33–44, 1995.
George A.E., de Leon M.J., Gentes C.I., Miller J., London E., Budzilovich G.N.: Leukoencephalopathy in normal and pathological ageing. Am. J. Neuroradiol. 7: 561–566, 1986.
Wallin A., Blennow K., Uhlemann C., Langstrom F., Gottfries C.G.: White matter low attenuations on CT in Alzheimer’s disease and vascular dementia; diagnostic and pathogenetic aspects. Acta Neurol. Scand. 80: 518–523, 1989.
Inzitari D., Diaz F., Fox A., Hachinski V.C., Steingart A., Lau C.: Vascular risk factors and leukoaraiosis. Arch. Neurol. 44: 42–47, 1987.
Amar K., Wilkock G.: Vascular dementia. BMJ 312: 227–231, 1996.
Blennow K., Wallin A., Uhlemann C., Gottfries C.G.: White-matter lesions on CT in Alzheimer patients: relation to clinical symptomatology and vascular factors. Acta Neurol. Scand. 83: 187–193, 1991.
Furuta A., Nobuyoshi I., Nischihara Y., Horie A.: Medullary arteries in aging and dementia. Stroke 22: 442–446, 1991.
Roman G.C.: Senile dementia of the Binswanger type: a vascular form of dementia in the elderly. JAMA 258: 1782–1788, 1987.
Pantoni L., Garcia J.H.: Pathogenesis of leukoaraiosis: a review. Stroke 28: 652–659, 1997.
Brun A., Englund E.: A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann. Neurol. 19: 253–262, 1986.
Tumimoto H., Akiguchi I., Suenaga T., Nishimura M., Wakita H., Nakamura S., Kimura J.: Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrov-ascular and Alzheimer’s disease patients. Stroke 27: 2069–2074, 1996.
Hann J., Rooss R.A., Algra P.R.: Hereditary cerebral haemorrhage with amyloidosis-Dutch type. Magnetic resonance imaging findings in 7 cases. Brain 113: 1251–1267, 1990.
Erkinjuntti T., Benavente O., Eliasziw M.: Diffuse vacuoliza-tion (spongiosis) and arteriosclerosis in the frontal white matter occurs in vascular dementia. Arch. Neurol. 53: 325–332, 1996.
Steward R.: Cardiovascular factors in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 65: 143–147, 1998.
Breteler M.M.B., Van Swieten J.C., Bots M.L., Grobbee D.E., Claus J.J., Van Den Hout J.H.W., Van Harskamp F., Tanghe H.L.J., De Jong P.T.V.M., Van Gijn J., Hofman A.: Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study. The Rotterdam Study. Neurology 44: 1246–1252, 1994.
Smowdon D.A., Greiner L.H., Mortimer J.A., Riley K.P., Greiner P.A., Markesbery W.R.: Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 277: 813–817, 1997.
Sparks D.L., Scheff S.W., Liu H.: Increased incidence of neurofibrillary tangles in non-demented individuals with hypertension. J. Neurol. Sci. 131: 162–169, 1995.
Strassburger T.L., Lee H.C., Daly E.M., Szezepanic J., Krasuski J.S., Mentis M.J., Salerno J.A., DeCarli C., Shapiro M.B., Alexander G.E.: Interactive effects of age and hypertension on volume of brain structures. Stroke 28: 1410–1417, 1997.
Brun A., Gustafson L., Samulsson S.M., Ericsson C.: Neuropathology of late life. Dementia 3: 125–130, 1992.
Hulette C., Nochlin D., McKeel D.: Clinical-neuropathologic findings in multi-infarct dementia: a report of six autopsied cases. Neurology 48: 668–672, 1997.
Desmond D.W.: Vascular dementia: a construct in evolution. Cerebrovasc. Brain Metab. Rev. 8: 296–325, 1996.
Nolan K.A., Lino M.M., Seligmann A.W., Blass J.P.: Absence of vascular dementia in an autopsy series from a dementia clinic. J. Am. Geriatr. Soc. 46: 597–604, 1998.
Nyenhuis D.L., Gorelick P.B.: Vascular dementia: a contemporary review of epidemiology, diagnosis, prevention and treatment. J. Am. Geriatr. Soc. 46: 1437–1448, 1998.
Hall E.D., Osteveen J.A., Dunn E.: Increased amyloid protein precursor and apolipoprotein E immunoreactivity in the selectively vulnerable hippocampus following transient forebrain ischemia in gerbils. Exp. Neurol. 135: 17–27, 1995.
Pluta R., Barcikowsa M., Janusezewki S.: Evidence of blood-brain barrier permeability/leakage for circulating human Alzheimer’s β-amyloid (1-42) peptide. Neuroreport 7: 1261–1265, 1996.
Sasaki N., Fakatsu R., Tsuzuki K., Yoshida T., Fujii N., Koike T., Wakayama I., Yanagihara R., Garruto R., Amano N., Makita Z.: Advanced glycation end products in Alzheimer’s disease and other neurodegenerative disease. Am. J. Pathol. 153: 1149–1155, 1998.
Forette F., Seux M.L., Staessen J.A., Thijs L., Birkenhager W.H., Babarskiene M.R., Babeanu S., Bossini A., Gil-Extremera B., Girerd X., Laks T., Lilov E., Moisseyev V., Tuomileto J., Vanhanen H., Webster J., Yodfat Y., Fagard R.: Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 352: 1347–1351, 1998.
Shep Cooperative Research Group: Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: Final results of the systolic hypertension in the Elderly Program (SHEP). JAMA 265: 3255–3264, 1991.
Parnetti L., Senin U., Mecocci P.: Cognitive enhancement therapy for Alzheimer’s disease: the way forward. Drugs 53: 752–768, 1997.
Gould R.J., Murphy K.M., Snyder S.H.: Autoradiographic localization of calcium channel antagonist receptors in rat brain with (3H) nitrendipine. Brain Res. 330: 217–223, 1985.
Barrett-Connor E.: Rethinking estrogen in the brain. J. Am. Geriatr. Soc. 46: 918–920, 1998.
Yaffe K., Grady D., Pressman A., Cummings S.: Serum estrogen levels, cognitive performance, and risk of cognitive decline in older community women. J. Am. Geriatr. Soc. 46: 816–821, 1998.
Trabucchi M.: Le demenze. UTET, Milano, 1998.
McGeer P.L., Schulzer M., McGeer E.G.: Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology 47: 425–432, 1996.
Rich J.B., Rasmusson D.X., Folstein M.F.: Non-steroidal anti-inflammatory drugs in Alzheimer’s disease. Neurology 45: 51–55, 1995.
Steward W.F., Kawas C., Corrada M.: Risk of Alzheimer’s disease and duration of NSAID use. Neurology 48: 626–632, 1997.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Iorio, A., Zito, M., Lupinetti, M. et al. Are vascular factors involved in Alzheimer’s disease? Facts and theories. Aging Clin Exp Res 11, 345–352 (1999). https://doi.org/10.1007/BF03339811
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03339811