Summary
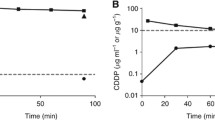
Total plasma platinum (TP) was measured over 21 days in 22 patients receiving a 6-hour intravenous infusion of the anticancer drug cisplatin 80 to 100 mg/m2. Cisplatin was administered as a single agent to 3 ovarian cancer patients, in combination with fluorouracil to 12 head and neck cancer patients, and with bleomycin and etoposide to 7 patients with teratoma of the testis. The mean (±SD) peak TP was 2.59 ±0.35 mg/L at the end of the infusion. Postinfusion TP declined triphasically, with a mean (±SD) half-life, plasma clearance, apparent volume of distribution at steady-state and mean residence time (MRT) of 11.7 ±2.3 days, 2.4 ±0.88 ml/min/m2, 49 ±13 L/m2 and 15 ±3.5 days, respectively. At 21 days all patients had measurable TP(0.32 ±0.11 mg/L), with 13 to 40% of the area under the concentration vs time curve from zero to infinity (AUC0-∞) remaining. 60% of the platinum dose remained to be excreted in urine, 4 days postinfusion, and the extrapolated mean MRT was 17.4 days. We conclude that a long TP terminal half-life is present in all patients, which will lead to platinum accumulation on consecutive cisplatin infusions at dose intervals of 3 weeks or less.
Similar content being viewed by others
References
Belt RJ, Himmelstein KJ, Patton TF, Bannister SJ, Sternson LA, et al. Pharmacokinetics of non-protein-bound platinum species following administration of cis-dichlorodiammineplatinum (II). Cancer Treatment Reports 63: 1515–1521, 1979
Bues-Charbit M, Gentet J, Bernard J, Breant V, Cano J, et al. Continuous infusion of high dose cisplatin in children: pharmacokinetics of free and total platinum. Journal of Cancer and Clinical Oncology 23: 1649–1652, 1987
Buice RG, Soloway MS. Platinum kinetics in patients treated with cis-dichlorodiammine platinum (II). Therapeutic Drug Monitoring 4: 293–296, 1982
Crom WR, Evans WE, Pratt CB, Senzer N, Denison M, et al. Cisplatin disposition in children and adolescents with cancer. Cancer Chemotherapy and Pharmacology 6: 95–99, 1981
DeConti RC, Toftness BR, Lange RC, Creasey WA. Clinical and pharmacological studies with cis-diamminedichloroplatinum (II). Cancer Research 33: 1310–1315, 1973
Erlichman C, Soldin SJ, Thiessen J, Sturheon JFG, Fine S. Disposition of total and free cisplatin on two consecutive treatment cycles in patients with ovarian cancer. Cancer Chemotherapy and Pharmacology 19: 75–79, 1987
Gandara DR, Perez EA, Denham A, Wiebe VJ, DeGregorio M. Pharmacokinetics of cisplatin in patients receiving interleukin-2-containing treatment regimes. Cancer Chemotherapy and Pharmacology 24: 135–136, 1989
Gershenson D, Wharton J, Herson J, Edward C, Rutledge F. Single agent cis-platinum therapy for advanced ovarian cancer. Obstetrics and Gynecology 58: 487–496, 1981
Gibaldi M, Perrier D (Eds). Pharmacokinetics, 2nd ed., Vol. 15, Dekker, New York, 1982
Gormley PE, Bull JM, LeRoy AF, Cysyk R. Kinetics of cisdichlorodiammineplatinum. Clinical Pharmacology and Therapeutics 5: 351–357, 1979
Griffiths H, Shelley MD, Fish RG. A modified pharmacokinetic model for platinum disposition in ovarian cancer patients receiving cisplatin. European Journal of Clinical Pharmacology 33: 67–72, 1987
Gullo JJ, Litterst CL, Maguire P, Sikic B, Hoth DF, Woolley PV. Pharmacokinetics and protein binding of cis-dichlorodiammine platinum (II) administered as a one hour or a twenty hour infusion. Cancer Chemotherapy and Pharmacology 5: 21–26, 1980
Hedegus L, Van Der Vijgh W, Klein I, Kerpel-Fronius S, Pinedo H. Chemical reactivity of cisplatin bound to human plasma proteins. Cancer Chemotherapy and Pharmacology 20: 211–212, 1987
Loehrer PT, Einhorn LH. Diagnosis and treatment, drugs 5 years later. Annals of Internal Medicine 100: 704–713, 1984
Murakami T, Inoue S, Sasaki K, Fujimoto T. Studies on age-dependent plasma platinum pharmacokinetics and ototoxicity of cisplatin. Selective Cancer Therapeutics 6: 145–151, 1990
Patton TF, Himmelstein KJ, Belt R, Bannister SJ, Sternson LA, et al. Plasma levels and urinary excretion of filterable platinum species following bolus injection and IV infusion of cisdichlorodiammineplatinum (II) in man. Cancer Treatment Reports 62: 1359–1362, 1978
Reddel RR, Kefford RF, Grant JM, Coates AS, Fox RM, et al. Ototoxicity in patients receiving cisplatin: importance of dose and method of drug administration. Cancer Treatment Reports 66: 19–23, 1982
Reece P, Stafford I, Russell J, Khan M, Gill P. Creatinine clearance as a predictor of ultrafilterable platinum disposition in cancer patients treated with cisplatin: relationship between peak ultrafilterable platinum plasma levels and nephrotoxicity. Journal of Clinical Oncology 5: 304–309, 1987
Shelley MD. Studies on platinum drugs in ovarian cancer patients. Ph.D. Thesis, University of Wales, 1987
Smith PH, Taylor DM. Distribution and retention of the antitumour agent 195m Pt-cis-dichlorodiammine platinum (II) in man. Journal of Nuclear Medicine 15: 344–351, 1974
Stewart DJ, Mikhael NZ, Nair RC, Kacew S, Montpetit V, et al. Platinum concentrations in human autopsy tumour samples. American Journal of Clinical Oncology 11: 152–158, 1988
Takahashi K, Seki T, Nishikawa K, Minamide S, Iwabuchi M, et al. Antitumor activity and toxicity of serum protein-bound platinum formed from cisplatin. Japanese Journal of Cancer Research 76: 68–74, 1985
Tothill P, Klys HS, Matheson LM, McKay K, Smyth JF. The long-term retention of platinum in human tissues following the administration of cisplatin or carboplatin for cancer chemotherapy. European Journal of Cancer 28: 1358–1361, 1992
Vermorken JB, Van Der Vijgh WJF, Klein I, Gall H, Pinedo HM. Pharmacokinetics of free-platinum species following rapid, 3-hr and 24-hr infusions of cis-diamminedichloroplatinum (II) and its therapeutic implications. European Journal of Cancer and Clinical Oncology 18: 1069–1074, 1982
Vermorken JB, Van Der Vijgh WJF, Klein I, Hart AA, Gall HE, et al. Pharmacokinetics of free and total platinum species after short-term infusion of cisplatin. Cancer Treatment Reports 68: 505–513, 1984
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fish, R.G., Shelley, M.D., Badman, J. et al. Platinum Accumulation in Adult Cancer Patients Receiving Cisplatin. Drug Invest 7, 175–182 (1994). https://doi.org/10.1007/BF03257408
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03257408