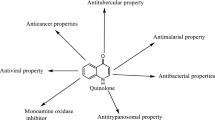
Abstract
Some new derivatives of 1,2,4-triazolo[2,3-a]benzimidazoles were synthesized through the reaction of 1,2-diaminobenzimidazole with carbon disulfide. The resulting 1,2,4-triazolo-[2,3-a]benzimidazole-2-thione intermediate was reacted with one equivalent of alkyl halides to give the corresponding 2-alkylthio derivatives, which were further alkylated through the reaction with another one equivalent of different alkyl halides to afford the target compounds; 1-alkyl-2-alkylthio-1,2,4-traizolo[2,3-a]benzimidazoles. On the other hand, the 1,2-disubstituted derivatives with two identical alkyl substituents were prepared by the reaction of 1,2,4-triazolo[2,3-a]benzimidazole-2-thione with two equivalents of the alkyl halides. The structures of the new compounds were assigned by spectral and elemental methods of analyses. The synthesized compounds were tested for their antibacterial and antifungal activities. Most of the tested compounds proved comparable results with those ofampicillin andfluconazole reference drugs. The study indicated that, the antibacterial as well as the antifungal activities of the test compounds were improved with increase in the bulkiness of the introduced alkyl groups. Also, some active antibacterial compounds were tested for their antimycobacterial activity. All the test compounds showed equipotent antitubercular activity as that ofINH as a reference drug.
Similar content being viewed by others
References
Canetti, G., Rist, N., and Grosset, J., Measure de la senseibilite du bacilli tuberculeux aux drogues antibacillaires par la methode des proportion,Rev. Tuber., 58, 111–114 (1983).
European Pharmacopoeia, 3rd ed., Councel of Europe, Strasbourg, p. 416 (1996a).
European Pharmacopoeia, 3rd ed., Councel of Europe, Strasbourg, p. 1259 (1996b).
Gülerman, N. N., Doðan, H. N., Rollas, S., Johansson, C., and Celik, C., Synthesis and structure elucidation of some new thioether derivatives of 1,2,4-triazoline-3-thione and their antimicrobial activities,IL Farmaco, 56, 953–958 (2001).
Hartman, W. W. and Dreger, E. E., Organic Synthesis, 2nd ed., collective volume II Blatt, A. H. editors, John Wiley and sons, Inc., New York, p. 150 (1943).
He, Y., Yang, J., Wu, B., Risen, L., and Swayze, E. E., Synthesis and biological evaluation of novel benzimidazoles as potential antibacterial agents.Bioorganic and Medicinal Chemistry Letters, 14, 1217–1220 (2004).
Savini, L., Chiasserini, L., Pellerano, C., Filippelli, W., and Falcone, G., Synthesis and pharmacological activity of 1,2,4-triazolo[4,3-a]quinolines.IL Farmaco, 56, 939–945 (2001).
Smith, D. M., Chemistry of Heterocyclic Compounds, Vol. 40, part I, Weissberger, A., editor, John Wiley and Sons, New York, p. 331, (1981).
Stecher, Paul, G., The Merck Index, 13 ed., Budavari, S., editor, Rahway, N. J., U.S.A., p. 203 (1989a).
Stecher, Paul, G., The merck Index, 13 ed., Budavari, S., editor, Rahway, N. J., U.S.A., p. 409 (1989b).
Stecher, Paul, G., The Merck Index, 13 ed., Budavari, S., editor, Rahway, N. J., U.S.A., p. 409 (1989b).
Stecher, Paul, G. The Merck Index, 13 ed., Budavari, S., editor, Rahway, N. J., U.S.A., p. 961 (1989c).
Stecher, Paul, G., The Merck Index, 13 ed., Budavari, S., editor, Rahway, N. J., U.S.A., p. 728 (1989d).
Stecher, Paul, G., The Merck Index, 13 ed., Budavari, S., editor, Rahway, N. J., U.S.A., p. 931 (1989e).
Stecher, Paul, G., The Merck Index, 13 ed., Budavari, S., editor, Rahway, N. J., U.S.A., p. 105 (1989f).
Stecher, Paul, G., The Merck Index, 13 ed., Budavari, S., editor, Rahway, N. J., U.S.A., pp. 974 (1989g).
Stecher, Paul, G., The Merck Index, 13 ed., Budavari, S., editor, Rahway, N. J., U.S.A., p. 659 (1989h).
William H., “Microbiological assay, An introduction to quantitative principles and evaluation”, Academic press, New York, (1977).
United state pharmacopoeia, 25th Asian Edition., Webcom limited, Toronto, pp. 662, 1529, 37, and 934 (2002a).
United state pharmacopoeia, 25th Asian Edition., Webcom limited, Toronto, p. 1377 (2002b).
United state pharmacopoeia, 25th Asian Edition., Webcom limited, Toronto, p. 47 (2002c).
Zeiger, A. V. and Joullié, M. M., Oxidation of 1,2-diaminobenzimidazole to 3-amino-1,2,4-benzotriazines.J. Org. Chem., 42, 542–545 (1977).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohamed, B.G., Hussein, M.A., Abdel-Alim, AA.M. et al. Synthesis and antimicrobial activity of some new 1-alkyl-2-alkylthio-1,2,4-triazolobenzimidazole derivatives. Arch Pharm Res 29, 26–33 (2006). https://doi.org/10.1007/BF02977464
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02977464