Abstract
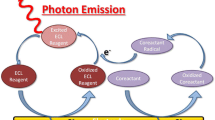
The effect of fluidic conditions on the bioluminescent detection of ATP in a microfluidic device was surveyed using homemade detector system. The bioluminescent reaction of ATP was directly affected by the retention time and flow rates of the solutions in this diffusion-based mixing and reaction system due to the laminar flow in the microchannel. ATP and enzyme solutions were separately injected into the microfluidic device at different flow rates through 3 inlet ports. The ATP solution was injected through the middle port, while the enzyme solution was injected in the two remaining ports. When the ratio of ATP to enzyme solution was fixed, the optimum flow rates of enzyme, ATP, and enzyme solution was 3.5, 8.0, and 3.5 μL/min, respectively. The optimal total flow rate was 15 μL/min. The detection limit for the concentration of ATP at optimal conditions was less than 10−7 M.
Similar content being viewed by others
References
Duffy, D. C., J. C. McDonald, O. J. A. Schueller, and G. M. Whitesides (1998) Rapid prototyping of microfluidic systems in poly(dimethylsiloxane).Anal. Chem. 70: 4974–4984.
Stachowiak, T. B., T. Rohr, E. F. Hilder, D. S. Peterson, M. Yi, F. Svec, and J. M. J. Frechet (2003) Fabrication of porous polymer monoliths covalently attached to the walls of channels in plastic microdevices.Electrophoresis 24: 3689–3693.
Li, C., Y. Yang, H. G. Craighead, and K. H. Lee (2005) Isoelectric focusing in cyclic olefin copolymer microfluidic channels coated by polyacrylamide using a UV photografting method.Electrophoresis 26: 1800–1806.
Sohn, Y.-S., J. Kai, and C. H. Ahn (2004) Protein array patterning on cyclic olefin copolymer (COC) for disposable protein chip.Sensor Lett. 2: 171–174.
Weigl, B. H., R. L. Bardell, and C. R. Cabrera, (2003) Lab-on-a-chip for drug development.Adv. Drug Deliv. Rev. 55: 349–377.
Brolin, S. E. and G. Wettermark (1992)Bioluminescence Analysis. VCH, New York, NY, USA.
Ahn, J.-M., B. C. Kim, and M. B. Gu (2006) Characterization ofgltA::luxCDABE fusion inEscherichia coli as a toxicity biosensor.Biotechnol. Bioprocess Eng. 11: 516–521.
Min, J. (2007) 17β-Estradiol-stimulated eNOS gene transcriptional activation is regulated through the estrogen-responsive element in eNOS promoter.Biotechnol. Bioprocess Eng. 12: 446–449.
Girotti, S., E. N. Ferri, S. Ghini, F. Fini, M. Musiani, G. Carrea, A. Roda, and P. Rauch (1997) Luminescent techniques applied to bioanalysis.Chem. Listy 91: 477–482.
Liu, B.-F., M. Ozaki, H. Hisamoto, Q. Luo, Y. Utsumi, T. Hattori, and S. Terabe (2005) Microfluidic chip toward cellular ATP and ATP-conjugated metabolic analysis with bioluminescence detection.Anal. Chem. 77: 573–578.
Kim, Y.-B., J.-H. Park, W.-J. Chang, Y.-M. Koo, E.-K. Kim, and J.-H. Kim (2006) Statistical optimization of the lysis agents for gram-negative bacterial cells in a microfluidic device.Biotechnol. Bioprocess Eng. 11: 288–292.
Tsukagoshi, K., M. Tahira, and R. Nakajima (2005) Capillary electrophoresis apparatus equipped with a bioluminescence detector using a batch-or flow-type detection cell.J. Chromatogr. A 1094: 192–195.
Kirner, T., P. Jaschinsky, and J. M. Kohler (2004) Spatially resolved detection of miniaturized reaction-diffusion experiments in chip reactors for educational purposes.Chem. Eng. J. 101: 163–169.
Baroud, C. N., F. Okkels, L. Menetrier, and P. Tabeling (2003) Reaction-diffusion dynamics: confrontation between theory and experiment in a microfluidic reactor.Phys. Rev. E 67: 060104-1–060104-4.
Effenhauser, C. S., G. J. M. Bruin, A. Paulus, and M. Ehrat (1997) Integrated capillary electrophoresis on flexible silicone microdevices: analysis of DNA restriction fragments and detection of single DNA molecules on microchips.Anal. Chem. 69: 3451–3457.
Whitesides, G. M., E. Ostuni, S. Takayama, X. Jiang, and D. E. Ingber (2001) Soft lithography in biology and biochemistry.Annu. Rev. Biomed. Eng. 3: 335–373.
Bishop, R. H. (2001)LabVIEW Student Edition 6i. Prentice Hall, Upper Saddle River, NJ, USA.
Salama, K., H. Eltoukhy, A. Hassibi, and A. E. Gamal (2004) Modeling and simulation of luminescence detection platforms.Biosens. Bioelectron. 19: 1377–1386.
Gomi, K., and N. Kajiyama (2001) Oxyluciferin, a luminescence product of firefly luciferase, is enzymatically regenerated into luciferin.J. Biol. Chem. 276: 36508–36513.
De Wet, J. R., K. V. Wood, D. R. Helinski, and M. DeLuca (1985) Cloning of firefly luciferase cDNA and the expression of active luciferase inEscherichia coli.Proc. Natl. Acad. Sci. USA 82: 7870–7873.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tran, TH., Chang, WJ., Kim, YB. et al. The effect of fluidic conditions on the continuous-flow bioluminescent detection of ATP in a microfluidic device. Biotechnol. Bioprocess Eng. 12, 470–474 (2007). https://doi.org/10.1007/BF02931342
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02931342