Summary
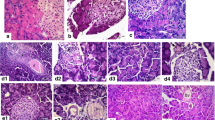
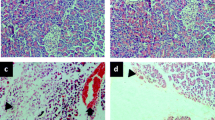
The aim of the present study was to determine the role of endogenous nitric oxide (NO) in pancreatic secretion in vivo and amylase release from pancreatic acini in vitro and in caerulein-induced acute pancreatitis in rats. Blockade of NO synthase byN G-nitro-L-arginine (L-NNA) (2.5 mg/kg iv) significantly reduced basal pancreatic protein secretion and that induced by the infusion of CCK (0.5 μg/kg-h), feeding, and the diversion of pancreatic juice in rats with pancreatic fistula. This inhibitory effect was partially reversed when L-arginine (50 mg/kg-h iv) was added to L-NNA. L-Arginine alone (50 mg/kg iv) did not affect basal or caerulein-induced pancreatic secretion. L-NNA, L-arginine, or their combination added in various concentrations to the incubation medium of dispersed acini failed to affect basal or secretagogue (caerulein or urecholine) stimulated amylase release. Infusion of caerulein (5 μg/kg-h) for 5 h produced histological changes of acute edematous pancreatitis accompanied by a marked increase in pancreatic protein content and about 50% reduction in tissue blood flow. L-NNA alone also reduced the pancreatic blood flow and caused a significant increase in pancreatic weight and protein content. L-NNA significantly potentiated the inflammatory changes in the pancreas caused by caerulein. Addition of L-arginine enhanced the pancreatic blood flow and ameliorated the pancreatitis induced by caerulein alone or that combined with L-NNA. We conclude that NO is involved in the stimulation of pancreatic secretion in vivo and exhibits a beneficial effect on pancreatitis, probably by improving the pancreatic blood flow.
Similar content being viewed by others
References
Aunes A, Semb LS. The effect of secretin and pancreozyminon pancreatic blood flow in the conscious andanesthetized dog.Acta Physiol Scand 1969; 76: 406–414.
Lenninger S. Effects of acetylcholine and papaverine onthe secretion and blood flow from the pancreas of the cat.Acta Physiol Scand 1973; 89: 260–268.
Beijer HJM, Brouwer FAS, Charbon GA. Time course andsensitivity of secretin stimulated pancreatic secretion andblood flow in the anesthetized dog.Scand J Gastroenterol 1979; 14: 295–300.
Konturek SJ, Pawlik W, Czarnobilski K, Gustaw P, Jaworek J, Beck G, Jendrella H. Effects of leukotriene C4on pancreatic secretion and circulation in dogs.Am JPhysiol 1988; 254: G849-G855.
Inoue K, Hosotani R, Tatemoto K, Yajima H, Tobe T. Effectof natural peptide YY on blood flow and exocrine secretionof pancreas in dogs.Dig Dis Sci 1988; 33: 828–832.
Pfeffer RB, Lazzarim-Robertson A Jr, Sadafi D, Mixter G Jr, Secoy CF, Hinton JW. Gradations of pancreatic, edematousthrough hemorrhagic experimentally produced bycontrolled injection of microspheres into the blood vesselsin dogs.Surgery 1962; 51: 764–769.
Broc PJ, Znidema GD, Cammeron JL. The role of ischemia in acute pancreatitis: studies with an isolated perfusedcanine pancreas.Surg 1981; 91: 377–383.
Popper HL, Nechela H, Russell KC. Transition of pancreaticedema to pancreatic necrosis.Surg Gynecol Obstet 1978; 87: 79–82.
Warshaw AL, O’Hara PJ. Susceptibility of the pancreas toischemic injury in shock.Ann Surg 1978; 188: 197–201.
Rose DM, Ranson JHC, Cunningham JN Jr, Spencer FC. Patterns of severe pancreatitis following cardiopulmonarybypass.Ann Surg 1984; 199: 168–172.
Panebianco AC, Scott SM, Part CH Jr, Takarot Echegaray HM. Acute pancreatitis following extracorporeal.AmThorac Surg 1970; 9: 562–568.
Harper SL, Pitts VH, Granger DN, Kvietys PR. Pancreatictissue oxygenation during secretory stimulation.Am JPhysiol 1986; 250G: 316–322.
Konturek SJ, Bilski J, Konturek PK, Cieszkowski M,Pawlik WW. Role of endogenous nitric oxide in the controlof canine pancreatic secretion and blood flow.Gastro-enterology 1993; 104: 896–902.
Holst JJ, Rassmussen TN, Schmidt P. Nitric oxide is animportant element in neurally induced pancreatic exocrinesecretion.Digestion 1992; 52: 90.
Palmer RMJ, Ferridge AG, Moneada S. Nitric oxiderelease accounts for the biological activity of arterial smoothmuscle by acetylocholine.Nature 1980; 288: 373–376.
Ignarro LJ, Buga GM, Wood KS, Byrus RE, Chandhuri G. Endothelium derived relaxing factor produced and releasedfrom artery and vein is nitric oxide.Proc Natl Acad Sci USA 1987; 84: 9265–9269.
Moore PK, Al Swayeh OA, Chong NWS, Evans RA,Gibson A. L-Ng/nitroarginine (L-NOARG), a novel, L-argi-nine-reversible inhibitor of endothelium-dependent vaso-dilator in vitro.Br J Pharmacol 1990; 99: 408–412.
Adler D, Hupp T, Kern HF. Course and spontaneousregression of acute pancreatitis in rats.Virchows Arch (A) 1979; 31: 282–294.
Robert A, Lum JT, Lancaster C, Olafsson AS, Kolbasa KP, Nezamis JE. Prevention by prostaglandins of caeru-lein-induced pancreatic in rats.Lab Invest 1989; 60: 677–691.
Konturek SJ, Dembinski A, Konturek PJ, Warzecha Z, Jaworek J, Gustaw P, Tomaszewska R, Stachura J. Role ofplatelet activating factor in pathogenesis of acute pancreatitisin rats.Gut 1992; 33: 1268–1274.
Prinz RA. Mechanisms of acute pancreatitis.Int J Pancreatol 1991; 9: 31–38.
Moncata S, Palmer RMJ, Higgs EA. Biosynthesis of nitricoxide from L-arginine. A pathway for the regulation of cellfunction and communication.Biochem Pharmacol 1989; 38: 1709–1715.
Konturek SJ, Krzyzek E, Bilski J. The importance ofgastric secretion in the feedback control of interdigestiveand postprandial pancreatic secretion in rats.Reg Pept 1991; 36: 85–97.
Amsterdam D, Solomon TE, Jamieson JD. Sequentialdissociation of exocrine pancreas into lobules, acini andindividual cells.Meth Cell Biol 1978; 20: 262–278.
Bernfeld P. Amylase alfa and beta.Meth Enzymol 1955; 5: 139–148.
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Proteinmeasurement with Folin phenol reagent.J Biol Chem 1951; 193: 265–275.
Molero X, Salas A, Guarner L, Malagelada J. Nitric oxidemodulates pancreatic responses to caerulein. Histologicaland biochemical improvement in acute pancreatitis.Digestion 1992; 52: 105.
Niederau C, Niederau M, Luthen R, Stohmeyer G, Ferrel LD, Gretell JH. Pancreatic exocrine secretion in acuteexperimental pancreatitis.Gastroenterology 1990; 99: 1120–1127.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Konturek, S.J., Szlachcic, A., Dembinski, A. et al. Nitric oxide in pancreatic secretion and hormone-induced pancreatitis in rats. Int J Pancreatol 15, 19–28 (1994). https://doi.org/10.1007/BF02924384
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02924384