Summary
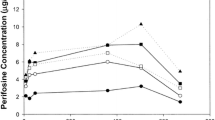
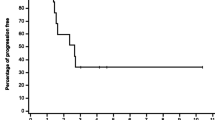
A phase I study of trimethylcolchicinic acid (TMCA) given orally once daily for 5 days every 3rd week was performed in 19 patients with advanced malignancies. Myelosuppression and mucositis were the major toxicities observed. Serum TMCA levels were monitored and appear to be useful in predicting toxicities. A partial response was seen in one lymphoma patient and stabilization of disease was noted in one patient each with prostatic and ovarian cancer.
Similar content being viewed by others
References
Amoroso EC (1935) Colchicine and tumor growth. Nature 135: 226–267
Brossi A, Yeh HJ, Chrzanowska M, Wolff J, Hamel E, Lin MC, Quin F, Suffness M, Silverton J (1988) Colchicine and its analogues: recent findings. Med Res Rev 84: 77–94
Goldberg B, Ortega LG, Goldin A, Ullyot G, Scheonbacj EB (1950) Studies on colchicine derivatives. Cancer 3: 124–129
Gomez GA, Sokal JE, Aungst CW (1970) Chemotherapy of the terminal phase of chronic myelocytic leukemia with colchicine derivations and purine analogs. Leuk Res: 1–6
Ko RJ, Li WY, Koda RT (1990) Determination of antimitotic agentsN-desacetylcolchicine, demecolcine and colchicine in serum and urine. J Chromatogr 525(2): 411–418
Lessner H, Johnson U, Loen V, Larsen W (1963) Primary clinical experience with trimethylcolchicinic acid etherd-tartrate (YMCA) in various malignancies. Cancer Chemother Rep 27: 33–38
Southwest Oncology Group (1989) Report of studies. SW Oncology Group Center, Washington
Stolinsky DC, Jacobs EM, Bateman JR, Hazen JG, Kuzma JW, Wood DA, Stainfeld JL (1967) Clinical trial of TMCA in advanced cancers. Cancer Chemother Rep 51: 25–34
Stolinsky DC, Jacobs EM, Braunwald J, Bateman JR (1973) Further study of TMCA in patients with malignant melanoma. Cancer Chemother Rep 56: 263–265
Stolinsky DC, Jacobs EM, Irwin LE, Pajak TF, Bateman JR (1976) Effect of trimethylcolchicinic acid methyl etherd-tartrate (TMCA) on Hodgkin’s and non-Hodgkin’s lymphoma. Oncology 3: 152–153
Stolinsky DC, Weiner JM, Bateman JR (1980) Rotational therapy with colchine analog in chronic granulocytic leukemia. Oncology 37: 62–63
Author information
Authors and Affiliations
Additional information
Supported in part by NIH DRR GCRC grant MOI RR-43
Rights and permissions
About this article
Cite this article
Hu, E., Ko, R., Koda, R. et al. Phase I toxicity and pharmacology study of trimethylcolchicinic acid in patients with advanced malignancies. Cancer Chemother Pharmacol 26, 359–364 (1990). https://doi.org/10.1007/BF02897294
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02897294