Abstract
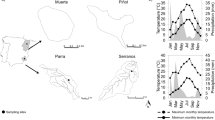
The spatial and temporal variations of meiofaunal communities in mangrove systems were examined. Replicated cores were taken in mudflats between prop roots ofRhizophora mangle at five locations within the Gulf of Batabanó, Cuba, during 3 mo. There was a clear seasonality in the water column, but measured abiotic variables did not show obvious relations with meiofaunal patterns. The magnitude of change in salinity for each location appears to influence the meiofauna more than absolute values per se. The meiofauna from southern Pinar del Rio showed a higher variation in community structure, suggesting higher levels of stress in comparison with locations in eastern Isla, possibly due to the presence of human settlements, runoff from land, and apparent deterioration of mangroves. The considerable variation in the density and community structure estimates on global (geographical regions) and local (locations in the Gulf of Batabanó) scales could be caused by the high spatial variability in the mangrove microenvironment, coupled with associated methodological differences in the sampling. There was a low density of meiofauna (mean: 101 animals 10 cm−2) compared to other shallow tropical habitats. Mangroves from subtropical and temperate regions showed consistently higher meiofaunal densities than tropical mangroves, but causes of this putatively latitudinal pattern require further study. Future strategies for meiofaunal studies in mangrove systems should increase the temporal and spatial replication, include designed field experiments to test ecological hypotheses, and apply a species level approach with regards to nematode assemblages.
Similar content being viewed by others
Literature Cited
Alongi, D. M. 1987a. Inter-estuary variation and intertidal zonation of free-living nematodes communities in tropical mangrove systems.Marine Ecology Progress Series 40:103–114.
Alongi, D. M. 1987b. Intertidal zonation and seasonality of meiobenthos in tropical mangrove estuaries.Marine Biology 95:447–458.
Alongi, D. M. 1987c. The influence of mangrove derived tannins on intertidal meiobenthos in tropical estuaries.Oecologia 71: 537–540.
Alongi, D. M. 1988. Microbial-meiofaunal interrelationships in some tropical intertidal sediments.Journal of Marine Research 46:349–365.
Alongi, D. M. 1989. The role of soft-bottom benthic communities in tropical mangroves ecosystems.Review of Aquatic Sciences 1:243–280.
Alongi, D. M. 1990. Community dynamics of free-living nematodes in some tropical mangrove and sandflat habitats.Bulletin of Marine Sceinces 46:358–373.
Alongi, D. M. andP. Christoffersen. 1992. Benthic infauna and organism-sediment relations in a shallow, tropical coastal area: Influence of outwelled mangrove detritus and physical disturbance.Marine Ecology Progress Series 81:229–245.
Baisre, J. 2001. Chronicle of Cuban marine fisheries (1935–1995). Trend analysis and fisheries potential.FAO Fisheries Technical Papers 394:1–26.
Clarke, K. R. andR. M. Warwick. 2001. Change in Marine Communities. An Approach to Statistical Analysis and Interpretation, 1st edition. PRIMER-E. Plymouth, U.K.
Coull, B. C. 1990. Are members of the meiofauna food for higher trophic levels?Transactions of the American Microscopy Society 109:233–246.
Coull, B. C. 1999. Role of meiofauna in estuarine soft-bottom habitats.Australian Journal of Ecology 24:327–343.
Dernie, K. M., M. J. Kaiser, E. A. Richardson, andR. M. Warwick. 2003. Recovery of soft sediment communities and habitats following physical disturbance.Journal of Experimental Marine Biology and Ecology 285–286:415–434.
Dierksmeier, G., R. Hernández, P. Moreno, K. Martinez, and C. Ricardo. 1996. Organochlorine pesticides in sediment and biota in the coastal region of the south of the Pinar del Río province, Cuba, p. 343–347.In Proceedings of Symposium Environmental Behavior of Crop Protection Chemicals, International Atomic Energy Agency, Vienna, Austria.
Dye, A. H. 1983a. Composition and seasonal fluctuation of meiofauna in a southern African mangrove estuary.Marine Biology 73:165–170.
Dye, A. H. 1983b. Oxygen consumption by sediments in a southern African mangrove swamp.Estuarine Coastal and Shelf Science 17:473–478.
Elliot, J. M. 1971. Some methods for the statistical analysis of samples of benthic invertebrates. Freshwater Biological Association. No. 25. Freshwater Biological Association, Westmorland, U.K.
Esteves, A. M. andV. M. A. P. Da Silva. 1998. The behavior of sugar flotation technique in meiofauna extraction from different sand types.Tropical Ecology 39:283–284.
Field, C. D. 1999. Charter for mangroves, p. 2–14.In A. Yañez-Arancibia and A. L. Lara-Domínguez (eds.), Mangrove Ecosystems in Tropical America. Union International for Conservation of Nature, Costa Rica: and National Oceanic and Atmospheric Administration, Veracruz, Mexico.
Gee, M. J. andP. J. Somerfield. 1997. Do mangrove diversity and leaf litter decay promote meiofaunal diversity?Journal of Experimental Marine Biology and Ecology 218:13–33.
Gwyther, J. 2000. Meiofauna in phytal-based and sedimentary habitats of a temperate mangrove ecosystem-A preliminary survey.Proceedings of the Royal Society of Victoria 112:137–151.
Gwyther, J. andP. G. Fairweather. 2002. Colonisation by epibionts and meiofauna of real and mimic pneumatophores in a cool temperate mangrove habitat.Marine Ecology Progress Series 229:137–149.
Gwyther, J. andP. G. Fairweather. 2005. Meiofaunal recruitment to mimic pneumatophores in a cool-temperate mangrove forest: Spatial context and biofilm effects.Journal of Experimental Marine Biology and Ecology 317:69–85.
Heip, C., M. Vincx, andG. Vranken. 1985. The ecology of marine nematodes.Oceanography and Marine Biology: An Annual Review 23:399–489.
Hodda, M. andW. L. Nicholas. 1985. Meiofauna associated with mangroves in the Hunter River estuary and Fulleton Cove, south-eastern Australia.Australian Journal of Marine and Fresheater Research 36:41–50.
Hodda, M. andW.L. Nicholas. 1986a. Nematode diversity and industrial pollution in the Hunter River estuary, NSW. Australia.Marine Pollution Bulletin 17:251–255.
Hodda, M. andW. L. Nicholas. 1986b. Temporal changes in littoral meiofauna from the Hunter River estuary.Australian Journal of Marine and Fresheater Research 37:729–741.
Hogarth, P. J. 1999. The Biology of Mangroves, 1st edition. Oxford University Press, New York.
Hopper, B. E., J. W. Fell andR. C. Cefalu. 1973. Effect of temperature on life cycles of nematodes associated with the mangrove (Rhizophora mangle) detritus system.Marine Biology 23:293–296.
Lalana-Rueda, R. andF. Gosselck. 1986. Investigations of the benthos of mangrove coastal lagoons in southern Cuba.Internationale Revue der gesamte Hydrobiologie 71:779–794.
Lambshead, P. J. D. andM. Hodda. 1994. The impact of disturbance on measurements of variability in marine nematode populations.Vie et Milieu 44:21–27.
Nicholas, W. L., J. A. Elek, A. C. Stewart, andT. G. Marples. 1991. The nematode fauna of a temperate Australian mangrove mudflat; Its population density, diversity and distribution.Hydrobiologia 209:13–27.
Olafsson, E. 1995. Merobenthos in mangrove areas in eastern Africa with emphasis on assemblage structure of free-living marine nematodes.Hydrobiologia 312:47–57.
Ólafsson, E., S. Carlstrom, andS. G. M. Ndaro. 2000. Meiobenthos of hypersaline tropical mangrove sediment in relation to spring tide inundation.Hydrobiologia 426:57–64.
Phillips, B. F., R. Cruz, N. Caputi, andR. S. Brown. 2000. Predicting the catch of spiny lobster fisheries, p. 357–375.In B. F. Phillips and J. Kittaka (eds.), Spiny Lobster: Fisheries and Culture. 2nd edition. Fishing News Books, Oxford, U.K.
Piñeiro, R. 2003. Bases para el manejo integrado del recurso langosta (Panulirus argus) en la zona costera sur de Pinar del Río. M.S. Thesis, Center for Marine Research, University of Havana, Havana, Cuba.
Proches, S. andD. J. Marshal. 2002. Algal growth and sediment deposition as determinants of distribution and abundance in mangrove pneumatophore arthropods.Journal of the Marine Biological Association of the United Kingdom 82:937–942.
Proches, S., D. J. Marshall, K. Ugrasen, andA. Ramcharan. 2001. Mangrove pneumatophore arthropod assemblages and seasonality patterns.Journal of the Marine Biological Association of the United Kingdom 81:545–552.
Rodríguez, J. P. andJ. E. Rodríguez. 1983. Las mareas de las coastas cubanas. Reporte de Investigación Instituto de Oceanología 6:1–37.
Rodriguez-Otero, G. 2001. Los asentamientos humanos, el uso de la tierra y los cambios globales en Cuba, Programa National de Cambios Globales. esProyecto 0130429. IPF, La Habana, Cuba.
Schmid-Araya, J. M. andP. E. Schmid. 2000. Trophic relationships: Integrating meiofauna into a realistic benthic food web.Freshwater Biology 44:149–163.
Somerfield, P. J., J. M. Gee, andC. Aryuthaka. 1998. Meiofaunal communities in a Malaysian mangrove forest.Journal of the Marine Biological Association of the United Kingdom 78:717–732.
Thiel, H. 1966. Quantitative untersuchungen über die meiofauna des tiefseebodens.Sonderband II:131–148.
Tietjen, J. H. andD. M. Alongi. 1990. Population growth and effects of nematodes on nutrient regeneration and bacteria associated with mangrove detritus from northeastern Queensland (Australia).Marine Ecology Progress: Series 68:169–180.
Zhou, H. 2001. Effects of leaf litter addition on meiofaunal colonization of azoic sediments in a subtropical mangrove in Hong Kong.Journal of Experimental Marine Biology and Ecology 256:99–121.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armenteros, M., Martín, I., Williams, J.P. et al. Spatial and temporal variations of meiofaunal communities from the Western Sector of the Gulf of Batabanó, Cuba. I. Mangrove systems. Estuaries and Coasts: J ERF 29, 124–132 (2006). https://doi.org/10.1007/BF02784704
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02784704