Abstract
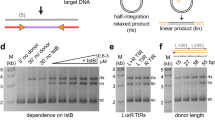
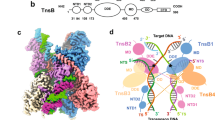
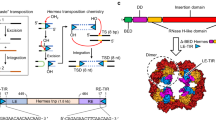
Assembly of the functional tetrameric form of Mu transposase (MuA protein) at the two att ends of Mu depends on interaction of MuA with multiple att and enhancer sites on supercoiled DNA, and is stimulated by MuB protein. The N-terminal domain I of MuA harbours distinct regions for interaction with the att ends and enhancer; the C-terminal domain III contains separate regions essential for tetramer assembly and interaction with MuB protein (IIIα and IIIβ, respectively). Although the central domain II (the ‘DDE’ domain) of MuA harbours the known catalytic DDE residues, a 26 amino acid peptide within IIIα also has a non-specific DNA binding and nuclease activity which has been implicated in catalysis. One model proposes that active sites for Mu transposition are assembled by sharing structural/catalytic residues between domains II and III present on separate MuA monomers within the MuA tetramer. We have used substrates with altered att sites and mixtures of MuA proteins with either wild-type or altered att DNA binding specificities, to create tetrameric arrangements wherein specific MuA subunits are nonfunctional in II, IIIα or IIIβ domains. From the ability of these oriented tetramers to carry out DNA cleavage and strand transfer we conclude that domain IIIα or IIIβ function is not unique to a specific subunit within the tetramer, indicative of a structural rather than a catalytic function for domain III in Mu transposition.
Similar content being viewed by others
Abbreviations
- HMK:
-
Heart muscle kinase
- DEP:
-
double-end strand transfer products
- SEP:
-
single-end strand transfer products
References
Aldaz H, Schuster E and Baker T A 1996 The interwoven architecture of the Mu transposase couples DNA synapsis to catalysis;Cell 85 257–269
Baker T A and Luo L 1994 Identification of residues in the Mu transposase essential for catalysis;Proc. Natl. Acad. Sci. USA 91 6654–6658
Baker T A, Mizuuchi M and Mizuuchi K 1991 MuB protein allosterically activates strand transfer by the transposase of phage Mu;Cell 65 1003–1013
Bolland S and Kleckner N 1996 The three chemical steps of Tn10/IS10 transposition involve repeated utilization of a single active site;Cell 84 223–233
Chaconas G, Lavoie B D and Watson M A 1996 DNA Transposition—jumping gene machine, some assembly required;Curr.Biol. 7 817–820
Chen J-W, Lee J and Jayaram M 1992 DNA cleavage in trans by the active site tyrosine during Flp recombination: switching protein partners before exchanging strands;Cell 69 647–658
Craigie R and Mizuuchi K 1987 Transposition of Mu DNA: joining of Mu to target DNA can be uncoupled from cleavage at the ends of Mu;Cell 51 493–501
Davies D R, Goryshin I Y, Reznikoff W S and Rayment I 2000 Three-dimensional structure of the Tn5 synaptic complex transposition intermediate;Science 289 77–85
Grindley N D and Leschziner A E 1995 DNA transposition: from a black box to a colour monitor;Cell 83 1063–1066
Harshey R M and Cuneo S 1986 Carboxyl-terminal mutants of phage Mu transposase;J. Genet. 65 159–174
Jiang H, Yang J-Y and Harshey R M 1999 Criss-crossed interactions between the enhancer and the att sites of phage Mu during DNA transposition;EMBO J. 18 3845–3855
Kim K, Namgoong S-Y, Jayaram M and Harshey R M 1995 Step-arrest mutants of phage Mu transposase. Implications in DNA-protein assembly, Mu end cleavage, and strand transfer;J. Biol. Chem. 270 1472–1479
Krementsova E, Giffin M J, Pincus D and Baker T A 1998 Mutational analysis of the Mu transposase. Contributions of two distinct regions of domain II to recombination;J. Biol. Chem. 273 31358–31365
Kruklitis R, Welty D J and Nakai H 1996 ClpX protein ofEscherichia coli activates bacteriophage Mu transposase in the strand transfer complex for initiation of Mu DNA synthesis;EMBO J. 15 935–944
Kuo C F, Zou A, Jayaram M, Getzoff E and Harshey R M 1991 DNA-protein complexes during attachment-site synapsis in Mu DNA transposition;EMBO J. 10 1585–1591
Lavoie B D and Chaconas G 1995 Transposition of phage Mu DNA;Curr. Top. Microbiol. Immunol. 204 83–102
Leung P and Harshey RM 1991 Two mutations of phage Mu transposase that affect strand transfer or interactions with B protein lie in distinct polypeptide domains;J. Mol. Biol. 219 189–199
Levchenko I, Luo L and Baker T A 1995 Disassembly of the Mu transposase tetramer by the ClpX chaperone;Genes Dev. 9 2399–2408
Levchenko I, Yamauchi M and Baker T A 1997 ClpX and MuB interact with overlapping regions of Mu transposase: implications for control of the transposition pathway;Genes Dev. 11 1561–1572
Li B L, Langer J A, Schwartz B and Pestka S 1989 Creation of phosphorylation sites in proteins: construction of a phosphorylatable human interferon alpha;Proc. Natl. Acad. Sci. USA 86 558–562
Maxwell A, Craigie R and Mizuuchi K 1987 B protein of bacteriophage Mu is an ATPase that preferentially stimulates intermolecular DNA strand transfer;Proc. Natl. Acad. Sci. USA. 84 699–703
Mizuuchi K 1992 Polynucleotidyl transfer reactions in transpositional DNA recombination;Annu. Rev. Biochem. 61 1011–1051
Mizuuchi M, Baker T A and Mizuuchi K 1995 Assembly of phage Mu transpososomes: cooperative transitions assisted by protein and DNA scaffolds;Cell 83 375–385
Mizuuchi M and Mizuuchi K 1993 Target site selection in transposition of phage Mu;Cold Spring Harbor Symp. Quant. Biol. 58 515–523
Naigamwalla D Z and Chaconas G 1997 A new set of Mu DNA transposition intermediates: alternate pathways of target capture preceding strand transfer;EMBO J. 16 5227–5234
Nakayama C, Teplow D B and Harshey RM 1987 Structural domains in phage Mu transposase: identification of the sitespecific DNA-binding domain;Proc. Natl. Acad. Sci. USA 84 1809–1813
Namgoong S-Y and Harshey R M 1998 The same two monomers within a MuA tetramer provide the DDE domains for the strand cleavage and strand transfer steps of transposition;EMBO J. 17 3775–3785
Namgoong S-Y, Jayaram M, Kim K and Harshey R M 1994 DNA-protein cooperativity in the assembly and stabilization of Mu strand transfer complex. Relevance of DNA phasing and att site cleavage;J. Mol. Biol. 238 514–527
Namgoong S-Y, Kim K, Saxena P, Jayaram M, Giedroc D P and Harshey R M 1998a Mutational analysis of domain IIβ of bacteriophage Mu transposase: domains IIα and IIβ belong to different catalytic complementation groups;J. Mol. Biol. 275 221–232
Namgoong S-Y, Sankaralingam S and Harshey R M 1998b Altering the DNA-binding specificity of Mu transposasein vitro;Nucleic Acids Res. 26 3521–3527
Rice P and Mizuuchi K 1995 Structure of the bacteriophage Mu transposase core: a common structural motif for DNA transposition and retroviral integration;Cell 82 209–220
Sarnovsky R J, May E W and Craig N L 1996 The Tn7 transposase is a heteromeric complex in which DNA breakage and joining activities are distributed between different gene products;EMBO J. 15 6348–6361
Savilahti H and Mizuuchi K 1996 Mu Transpositional Recombination: donor DNA cleavage and strand transfer in trans by the Mu transposase;Cell 85 271–280
Surette M G, Harkness T and Chaconas G 1991 Stimulation of the Mu A protein-mediated strand cleavage reaction by the Mu B protein, and the requirement of DNA nicking for stable type 1 transpososome formation.In vitro transposition characteristics of mini-Mu plasmids carrying terminal base pair mutations;J. Biol. Chem. 266 3118–3124
Watson M A and Chaconas G 1996 Three-site synapsis during Mu DNA transposition: A critical intermediate preceding engagement of the active site;Cell 85 435–445
Wu Z and Chaconas G 1994 Characterization of a region in phage Mu transposase that is involved in interaction with the Mu B protein;J. Biol. Chem. 269 28829–28833
Wu Z and Chaconas G 1995 A novel DNA binding and nuclease activity in domain III of Mu transposase: evidence for a catalytic region involved in donor cleavage;EMBO J. 14 3835–3843
Yamauchi M and Baker T A 1998 An ATP-ADP switch in MuB controls progression of the Mu transposition pathway;EMBO J. 17 5509–5518
Yang J-Y, Jayaram M and Harshey R M 1996 Positional information within the Mu transposase tetramer: catalytic contributions of individual monomers;Cell 85 447–455
Yang J-Y, Kim K, Jayaram M and Harshey R M 1995 Domain sharing model for active site assembly within the Mu a tetramer during transposition: the enhancer may specify domain contributions;EMBO. J. 14 2374–2384
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mariconda, S., Namgoong, SY., Yoon, KH. et al. Domain III function of Mu transposase analysed by directed placement of subunits within the transpososome. J Biosci 25, 347–360 (2000). https://doi.org/10.1007/BF02703788
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02703788