Abstract
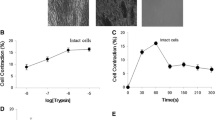
The mechanisms responsible for the mobilisation of Ca2+ from intracellular stores sensitive to inositol trisphosphate (InsP 3) were studied in saponin-permeabilised human myometrial cells in which the sarcoplasmic reticulum was pre-loaded with45Ca2+. InsP 3-induced45Ca2+ release was measured over the InsP 3 concentration range of 100 nM to 100 μM and showed a graded response. InsP 3-induced45Ca2+ release was inhibited by heparin (20–40 μg/ml) but not significantly affected by caffeine. The Ca2+ sensitivity of InsP 3-induced Ca2+ release was measured under conditions which were designed to exclude interference with Ca2+ released by the ryanodine receptor/channel complex. The data showed a bell-shaped relationship with the InsP 3 receptor (InsP 3R) functional at 10 nM, becoming maximally activated at 300 nM but inhibited at 10 μM Ca2+. Messenger RNA encoding for three isoforms of InsP 3R, type I, II and type III, was shown to be present. The relative expression levels of these messengers were obtained by ratio-PCR analysis and the levels of expression of the different isoforms were found to differ between individual patients.
Similar content being viewed by others
References
Arnaudeau S, Lepetre N, Mironneau J (1994) Oxytocin mobilizes calcium from a unique heparin-sensitive and thapsigargin-sensitive store in single myometrial cells from pregnant rat. Pflügers Arch 428:51–59.
Benevolensky D, Moraru II, Watras J (1994) Micromolar calcium decreases the affinity of inositol trisphosphate receptor in vascular smooth muscle. Biochem J 299:631–636
Berridge MJ (1993) Inositol trisphosphate and calcium signalling. Nature 361:315–325
Bezprozvanny I, Watras J, Ehrlich BE (1991) Bell-shaped calcium-response curves of Ins(1,4,5)P 3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351:751–754
Blondel O, Bell GI, Moody M, Miller RJ, Gibbons SJ (1994) Creation of an inositol 1,4,5-trisphosphate-sensitive Ca2+ store in secretory granules of insulin-producing cells. J Biol Chem 269:27167–27170
Blondel O, Moody MM, Depaoli AM, Sharp AH, Ross CA, Swift H, Bell GI (1994) Localization of inositol trisphosphate receptor subtype 3 to insulin and somatostatin secretory granules and regulation of expression in islets and insulinoma cells. Proc Natl Acad Sci USA 91:7777–7781
Cathala G, Savouret JF, Mendez B, West BL, KarinM, Martial JA, Baxter JD (1983) A method for isolation of intact, translationally active ribonucleic acid. DNA 2:329–335
Coronado R, Morrissette J, Sukhareva M, Vaughan DM (1994) Structure and function of ryanodine receptors. Am J Physiol 266:C1485–C1504
Danoff SK, Ferris CD, Donath C, Fischer G, Munemitsu S, Ullrich A, Snyder SH Ross CA (1991) Inositol 1,4,5-trisphosphate receptors: distinct neuronal and nonneuronal forms derived by alternative splicing differ in phosphorylation. Proc Natl Acad Sci USA 88:2951–2955
De Smedt H, Missiaen L, Parys JB, Bootman MD, Mertens L, Vandenbosch L, Casteels R (1994) Determination of the relative amounts of inositol trisphosphate receptor messenger-RNA isoforms by ratio polymerase chain-reaction. J Biol Chem 269:21691–21698
Enyedi P, Szabadkai G, Horvath A, Szilagyi L, Graf L, Spat A (1994) Inositol 1,4,5-trisphosphate receptor subtypes in adrenal glomerulosa cells. Endocrinology 134:2354–2359
Furuichi T, Kohda K, Miyawaki A, Mikoshiba K (1994) Intracellular channels. Curr Opin Neurobiol 4:295–303
Harnick DJ, Jayaraman, T, Ma Y, Mulieri P, Go LO, Marks AR (1995) The human type I inositol 1,4,5-trisphosphate receptor from T lymphocytes. J Biol Chem 270:2833–2840
Iino M (1989) Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol 194:363–383
Iino M, Endo M (1992) Calcium-dependent immediate feedback control of inositol 1,4,5-trisphosphate-induced Ca2+ release. Nature 360:76–78
Itoh T, Kuriyama H, Suzuki H (1981) Excitation-contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol (Lond) 321:513–535
Lynn S, Morgan JM, Gillespie JI, Greenwell JR (1993) A novel ryanodine sensitive calcium release mechanism in cultured myometrial smooth muscle cells. FEBS Lett 330:227–230
Lynn S, Morgan JM, Lamb HK, Meissner G, Gillespie JI (1995) Isolation and partial cloning of ryanodine-sensitive Ca2+ release channel protein isoforms from human myometrial smooth muscle. FEBS Lett 372:6–12
Missiaen L, De Smedt H, Droogmans G, Casteels R (1992) Luminal Ca2+ controls the activation of the inositol 1,4,5-trisphosphate receptor by cytosolic Ca2+. J Biol Chem 267:22961–22966
Missiaen L, De Smedt H, Droogmans G, Casteels R (1992) Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature 357:599–602
Missiaen L, De Smedt H, Parys JB, Casteels, R (1994) Co-activation of inositol trisphosphate-induced Ca2+ release by cytosolic Ca2+ is loading dependent. J Biol Chem 269:7238–7242
Missiaen L, Parys JB, De Smedt H, Sienaert I, Bootman M, Casteels R (1996) In: Biswas BB, Biswas S (eds) Subcellular biochemistry myoinositol phosphates, phosphoinositides and signal transduction. Plenum, New York (in press)
Morgan JM, Gillespie JI (1995) The modulation and characterisation of the Ca2+ induced Ca2+ release mechanism in cultured human myometrial smooth muscle cells. FEBS Lett 369:295–300.
Morgan JM, Lynn S, Gillespie JI, Greenwell JR (1993) The induction of intracellular calcium activity in cultured human myometrial smooth muscle cells. Biochim Biophys Acta 1158:98–102
Nakagawa T, Shiota C, Okano H, Mikoshiba K (1991) Differential localisation of alternative spliced transcripts encoding inositol 1,4,5-trisphosphate receptors in mouse cerebellum and hippocampus:in situ hybridization study. J Neurochem 57:1807–1810
Nakagawa T, Okano H, Furuichi T, Aruga J, Mikoshiba M (1991) The subtypes of the mouse inositol 1,4,5-trisphosphate receptor are expressed in a tissue-specific and developmentally specific manner. Proc Natl Acad Sci USA 88:6244–6248
Newton CL, Mignery GA, Südhof TC (1994) Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP 3) receptors with distinct affinities for InsP 3. J Biol Chem 269:28613–28619
Oldershaw KA, Taylor CW (1993) Luminal Ca2+ increases the affinity of inositol 1,4,5-trisphosphate for its receptor. Biochem J 292:631–633
Parker I, Ivorra I (1991) Caffeine inhibits inositol trisphosphate mediated liberation of intracellular calcium inxenopus oocytes. J Physiol (Lond) 433:229–240
Parys JB, De Smedt H, Missiaen L, Bootman MD, Sienaert I, Casteels R (1995) Rat basophilic leukemia cells as a model system for inositol 1,4,5-trisphosphate receptor IV, a receptor of the type II family: functional comparison and immunological detection. Cell Calcium 17:239–249
Petersen OH, Petersen CCH, Kasai H (1994) Calcium and hormone action. Annu Rev Physiol 56:297–319
Ross CA, Danoff SK, Schell MJ, Snyder SH, Ullrich A (1992) Three additional inositol 1,4,5-trisphosphate receptors: molecular cloning and differential localization in brain and peripheral tissues. Proc Natl Acad Sci USA 89:4265–4269
Savineau JP, Mironneau J (1990) Caffeine acting on pregnant rat myometrium: analysis of its relaxant action and its failure to release Ca2+ from intracellular stores. Br J Pharmacol 99:261–266
Smith GL, Miller DJ (1985) Potentiometric measurements of stoichiometric and apparent affinity constants of EGTA for protons and divalent ions including calcium. Biochim Biophy Acta 839:287–299
Somlyo AP, Somlyo AV (1994) Signal transduction and regulation in smooth muscle. Nature 372:231–236
Sudhof TC, Newton CL, Archer BT III, Ushkaryov YA, Mignery CA (1991) Structure of a novel InsP3 receptor. EMBO J 10:3199–3206
Sugiyama T, Yamamoto-Hino M, Miyawaki A, Furuichi T, Mikoshiba K, Hasegawa M (1994) Subtypes of inositol 1,4,5-trisphosphate receptor in human hematopoietic cell lines: dynamic aspects of their cell-type specific expression. FEBS Lett 349:191–196
Taylor CW, Richardson A (1991) Structure and function of inositol trisphosphate receptors. Pharmacol Ther 51:97–137
Toescu EC, O'Neill SC, Petersen OH, Eisner DA (1992) Caffeine inhibits the agonist-evoked cytosolic Ca2+ signal in mouse pancreatic acinar cells by blocking inositol trisphosphate production. J Biol Chem 267:23467–23470
Wray S (1993) Uterine contraction and physiological mechanisms of modulation. Am J Physiol 264:C1–C18
Yamada N, Makino Y, Clark RA, Pearson DW, Mattei M-G, Guenet J-L, Ohama E, Fujino I, Mikoshiba K (1994) Human inositol 1,4,5-trisphosphate type-1 receptor, InsP3R1: structure, function, regulation of expression and chromosomal localization. Biochem J 302:781–790
Yamamoto H, van Breeman C (1986) Ca2+ compartments in saponin-skinned cultured vascular smooth muscle cells. J Gen Physiol 87:369–389
Yamamoto-Hino M, Sugiyama T, Hikichi K, Mattei MG, Hasegawa K, Sekine S, Sakurada K, Miyawaki A, Furuichi T, Hasegawa M, Mikoshiba K (1994) Cloning and characterization of human type 2 and type 3 inositol 1,4,5 trisphosphate receptors. Receptors Channels 2:9–22
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morgan, J.M., De Smedt, H. & Gillespie, J.I. Identification of three isoforms of the InsP 3 receptor in human myometrial smooth muscle. Pflugers Arch. 431, 697–705 (1996). https://doi.org/10.1007/BF02253832
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02253832