Abstract
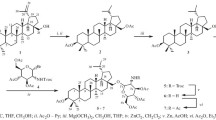
The two hydroxyls of yuccagenin can be glycosylated by Koenigs-Knorr condensation with acetobromorhammose in dichloroethane in the presence of mercuric cyanide. The bisrhamnoside of yuccagenin markedly lowers the cholesterol and triglyceride content in blood serum of healthy animals and animals with experimental hyperlipidemia.
Similar content being viewed by others
References
P. K. Kintya, G. V. Lazur'evskii, N. N. Balashova, et al.,Structure and Biological Activity of Steroidal Spirostan and Furostan-type Glycosides [in Russian], Shtiintsa, Kishinev (1987).
W. Koenigs and E. Knorr,Ber.,34, 957 (1901).
Albrecht,Liebigs Ann. Chem., 1429 (1977).
Yu. S. Vollerner,Spirostan and Furostan Steroids from Allium karataviense Rgl. Flowers [in Russian], Tashkent (1985).
N. K. Kochetkov, ed.,Methods of Carbohydrate Chemistry [in Russian], Moscow (1967), p. 123.
M. A. Khodzhaeva, Z. A. Khushbaktova, and N. S. Irismetova,Khim. Prir. Soedin., 731 (1998).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 3, pp. 242–243, May–June, 2000.
Rights and permissions
About this article
Cite this article
Pal'yants, N.S., Khushbaktova, Z.A., Pshenichnov, E.A. et al. Synthesis and biological activity of yuccagenin bisrhamnoside. Chem Nat Compd 36, 299–301 (2000). https://doi.org/10.1007/BF02238341
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02238341