Abstract
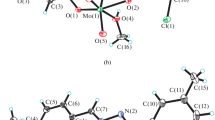
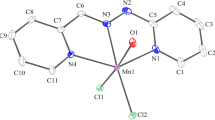
Several new complexes of dioxomolybdenum(VI) of the general formula [MoO2(L)S], whereL is the dianion of salicylaldehydep-hydroxybenzoylhydrazone andS denotes H2O, MeOH, py, PPh3, DMSO or DMF, were synthesized and characterized by elemental analysis, electronic UV-VIS and IR spectra, thermal analysis, molar conductivity and magnetic susceptibility measurements. Salicylaldehydep-hydroxybenzoylhydrazone participates in the coordination as a tridentate ligand with the ONO set of donor atoms. The complexes contain acis-MoO2 group and are of octahedral geometry. Complexes of the MoO2L type were also prepared by synthesis in CHCl3 solution and by isothermal heating of [MoO2(L)S] complexes. The MoO2L complex synthesized in CHCl3 solution has most probably a pentacoordinated structure while the complex obtained by isothermal heating of [MoO2(L)S] has a polymeric hexacoordinated structure.
Similar content being viewed by others
References
M. Coughlan, Ed., Molybdenum and Molybdenum-Containing Enzymes, Pergamon Press, New York 1980.
F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, 5th ed., Wiley Interscience, New York 1988.
E. I. Stiefel, Prog. Inorg. Chem., 22 (1977) 8.
B. Kamenar, Kem. Ind., 30 (1981) 373.
V. A. Kogan, V. V. Zelentsov, G. M. Larin and V. V. Lukov, Kompleksy Perekhodnykh Metallov s Gidrazonami, Nauka, Moskva 1990.
A. P. Grekov, Organicheskaya Khimia Hidrazina, Tehnika, Kiev 1966.
M. M. Jones, J. Am. Chem. Soc., 81 (1959) 3188.
W. C. Fernelius, K. Terada and B. E. Bryant, Inorg. Synth., 6 (1960) 147.
T. V. Troepol'skaya and E. N. Munin, Khimiya Gidrazonov, ed. Yu. P. Kitaev, Nauka, Moskva 1977.
R. H. Holm, G. W. Everett, Jr. and A. Chakravarty, Prog. Inorg. Chem., 7 (1966) 83.
A. Syamal and O. P. Singhal, Transition Met. Chem., 4 (1979) 179.
A. Syamal and M. R. Maurya, Transition Met. Chem., 10 (1985) 45.
A. Syamnal and D. Kumar, Transition Met. Chem., 7 (1982) 118.
O. A. Rajan and A. Chakravorty, Inorg. Chem., 20 (1981) 660.
K. Yamanouchi and S. Yamada, Inorg. Chim. Acta., 9 (1974) 161.
J. R. Dillworth, C. A. McAuliffe and B. J. Sayle, J. Chem. Soc. Dalton Trans, (1977) 849.
V. M. Leovac, I. Ivanovic, K. Andjelkovic and S. Mitrovska, J. Serb. Chem. Soc., 60 (1995) 1.
S. Bhattacharjee and R. Bhattacharyya, J. Chem. Soc. Dalton Trans, (1992) 1357.
A. Syamal and K. S. Kale, Inorg. Chem., 18 (1979) 992.
Author information
Authors and Affiliations
Additional information
The authors are grateful to the Serbian Republic Research Fund for financial support and to academician Prof. M. šušić for his interest in this work.
Rights and permissions
About this article
Cite this article
Ivanović, I., Andjelković, K., Leovac, V.M. et al. Transition-metal complexes with hydrazides and hydrazones. Journal of Thermal Analysis 46, 1741–1750 (1996). https://doi.org/10.1007/BF01980779
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01980779