Abstract
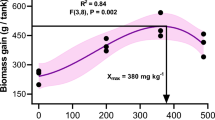
A study was conducted to determine the interrelationships between dietary ascorbic acid (AA) concentrations, brain neurotransmitter levels and weight gain in juvenile rainbow trout. At the end of 4 weeks and until the end of 12 weeks of feeding test diets of varying AA concentrations (0–320 mg AA/kg diet), increased weight gain was noted in fish fed the AA-free diet. However, by the end of 13 weeks and until the end of the experiment this phenomenon was no longer evident; instead the fish showed the more classical deficiency signs of anorexia and decreased weight gain. After 12 and 24 weeks, there were no significant differences in brain serotonin (5-HT), 5-hydroxyindoleacetic acid (5-HIAA), norepinephrine (NE) or dopamine (DA) between fish reared on the different test diets. However, after 12 weeks of feeding the test diets, brain 5-HT, brain AA and weight gain were significantly correlated with one another. No such relationships were found for brain NE or brain DA. After 24 weeks of feeding the diets, the relationships between brain 5-HT, brain AA and weight gain were no longer apparent. Similarly, after 24 weeks brain NE and DA were also unrelated to brain AA and weight gain. These results provide evidence that in very young rainbow trout, AA deficiency, brain 5-HT levels and weight gain were related. However in fish reared on the diets for 24 weeks these relationships were no longer evident.
Similar content being viewed by others
References cited
Aeschbacher, H.V. and Brown, R.G. 1972. Automated vitamin C analysis. Clin. Chem. 18: 965–967.
Akiyama, T., Murai, T. and Mori, K. 1986a. Role of tryptophan metabolites in inhibition of spinal deformity of chum salmon fry caused by tryptophan deficiency. Bull. Jap. Soc. Sci. Fish. 52: 1255–1259.
Akiyama, T., Murai, T. and Nose, T. 1986b. Oral administration of serotonin against spinal deformity. Bull. Jap. Soc. Sci. Fish. 52: 1249–1254.
Anderson, G.H. 1977. Regulation of protein intake by plasma amino acids.In Advances in Nutrition Research. Vol. 1. pp. 145–166. Edited by H.H. Draper. Plenum, New York.
Anderson, G.H. 1979. Control of protein and energy intake. Role of plasma amino acids and brain neurotransmitters. Can. J. Physiol. Pharmacol. 57: 1043–1057.
Basu, T.K. and Schorah, C.J. 1982. Vitamin C In Health and Disease. The Avi Publishing Company, Inc., Connecticut
Bligh, E.G. and Dyer, W.G. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917.
Cooper, J.R. 1961. The role of ascorbic acid in the oxidation of tryptophan to 5-hydroxytryptophan. Ann. N.Y. Acad. Sci. 92: 208–211.
Cooper, J.R., Bloom, F.E. and Roth, R.H. 1978. The Biochemical Basis of Neuropharmacology. Third Edition. Oxford University Press, New York.
Cooper, J.R. and Melcer, I. 1961. The enzymic oxidation of tryptophan to 5-hydroxytryptophan in the biosynthesis of serotonin. J. Pharmacol. Exp. Ther. 132: 265–268.
Essman, W.B. and Cooper, B.E. 1978. Serotonin and early development.In Serotonin in Health and Disease. Vol. II: Physiological Regulation and Pharmacological Action. pp. 69–157. Edited by W. B. Essman, Spectrum Publications, Inc. New York.
Fernstrom, J.P. 1985. Dietary effects on brain serotonin synthesis: Relationship to appetite regulation. Am. J. Clin. Nutr. 42: 1072–1082.
Freedland, R.A. 1963. Factors affecting the activityin vitro of the tryptophan-and phenylalanine-hydroxylating systems. Biochim. Biophys. Acta 73: 71–75.
Gal, E.M., Armstrong, J.C. and Ginsberg, B. 1966. The nature ofin vitro hydroxylation of L-tryptophan by brain tissue. J. Neurochem. 13: 643–654.
Gardiner, T.W., Armstrong-James, M., Caan, A.W., Wightman, R.M. and Rebec, G.V. 1985. Modulation of neostriatal activity by iontophoresis of ascorbic acid. Brain Res. 344: 181–185.
Grahame-Smith, D.G. 1967. The biosynthesis of 5-hydroxytryptamine in brain. Biochem. J. 105: 351–360.
Grahame-Smith, D.G. and Moloney, L. 1965. The subcellular localization, purification and properties of tryptophan 5-hydroxylase in brain. Biochem. J. 96: 66P-67P.
Halver, J.E., Ashley, L.M. and Smith, R.E. 1969. Ascorbic acid requirements of coho salmon and rainbow trout. Trans. Am. Fish. Soc. 98: 762–771.
Hilton, J.W. 1978. Ascorbic acid in the nutritionof rainbow trout (Salmo gairdneri R.) PhD Dissertation. University of Guelph, Guelph, Canada.
Hilton, J.W., Cho, C.Y. and Slinger, S.J. 1977. Evaluation of the ascorbic acid status of rainbow trout (Salmo gairdneri). J. Fish. Res. Board Can. 34: 2207–2210.
Hilton, J.W., Brown, R.G. and Slinger, S.J. 1979. The half-life and uptake of14C-1-ascorbic acid in selected organs of rainbow trout (Salmo gairdneri). Comp. Biochem. Physiol. A. 62: 427–432.
Hilton, J.W., Cho, C.Y. and Slinger, S.J. 1978. Effect of graded levels of supplemental ascorbic acid in practical diets fed to rainbow trout (Salmo gairdneri). J. Fish. res. Board Can. 35: 431–436
Hornig, D. 1975. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann. N.Y. Acad. Sci. 258: 103–118.
Horowitz, W., Chichilo, P. and Reynolds, H. (eds.). 1970. AOAC Methods, Official Method of Analysis of the Official Analytical Chemists. Eleventh edition. Association of Official Analytical Chemists, Washington, D.C.
Hughes, R.E., Hurley, R.J. and Jones, P.R. 1971. The retention of ascorbic acid by guinea-pig tissues. Br. J. Nutr. 26: 433–438.
Kaufman, S. and Friedman, S. 1965. Dopamine β-hydroxylase. Pharmacol. Rev. 17: 71–100.
Kitamura, S., Ohara, S., Suwa, T. and Nakagawa, K. 1965. Studies on vitamin requirements of rainbow trout,Salmo gairdneri I. On ascorbic acid. Bull. Jap. Soc. Sci. Fish. 31: 818–826.
Knaack, D., Shen, I., Salpeter, M. and Podleski, T.R. 1986. Selective effects of ascorbic acid on acetylcholine receptor number and distribution. J. Cell Biol. 102: 795–802.
Kuo, C., Hata, F., Yoshida, H., Yamatodani, A. and Wada, H. 1979. Effect of ascorbic acid on release of acetylcholine from synaptic vesicles prepared from different species of animals and release of noradrenaline from synaptic vesicles of rat brain. Life Sci. 24: 911–916.
Levine, M. 1986. Ascorbic acid specifically enhances dopamine-β-monooxygenase activity in resting and stimulated chromaffin cells. J. Biol. Chem. 261: 7347–7356.
Levine, M., Morita, K., Heldman, E. and Pollard, H.B. 1985a. Ascorbic acid regulation of norepinephrine biosynthesis in isolated chromaffin granules from bovine adrenal medulla. J. Biol. Chem. 260 15598–15603.
Levine, M., Morita, K. and Pollard, H. 1985b. Enhancement of norepinephrine biosynthesis by ascorbicacid in cultured bovine chromaffin cells. J. Biol. Chem. 260: 12942–12947.
Loizou, L.A. 1972. The postnatal ontogeny of monoamine-containing neurones in the central nervous system of the albino rat. Brain Res. 40: 395–418.
Lytle, L.D. 1977. Control of eating behaviour.In Nutrition and the Brain. Vol. 2. pp. 1–145. Edited by R.J. Wurtman and J.J. Wurtman. Raven Press, New York.
Mefford, I.N. 1981. Application of high performance liquid chromatography with electrochemical detection to neurochemical analysis: Measurement of catecholamines, serotonin and metabolites in rat brain. J. Neurosci. Methods 3: 207–224.
Mefford, I.N., Oke, A.F. and Adams, R.N. 1981. Regional distribution of ascorbate in human brain. Brain Res. 212: 223–226.
Menniti, F.S., Knoth, J. and Diliberto, E.J., Jr. 1986. Role of ascorbic acid in dopamine-β-hydroxylation. The endogenous enzyme cofactor and putative electron donor for cofactor regeneration. J. Biol. Chem. 261: 16901–16908.
Morita, K., Levine, M. and Pollard, H.B. 1986. Stimulatory effect of ascorbic acid on norepinephrine biosynthesis in digitonin-permeabilized adrenal medullary chromaffin cells. J. Neurochem. 46: 939–945.
Nakashima, Y., Sanada, H., Suzue, R. and Kawada, S. 1976. Role of ascorbic acid on tyrosine hydroxylase activity in the adrenal gland of guinea pig. J. Nutr. Sci. Vitaminol. 22: 355–363.
Nakashima, Y., Suzue, R., Sanada, H. and Kawada, S. 1970. Effect of ascorbic acid on hydroxylase activity. I. Stimulation of tyrosine hydroxylase and tryptophan-5-hydroxylase activities by ascorbic acid. J. Vitamin. 16: 276–280.
Nakashima, Y., Suzue, R., Sanada, H. and Kawada, S. 1972. Effect of ascorbic acid on tyrosine hydroxylase activityin vivo. Arch. Biochem. Biophys. 152: 515–520.
Steel, R.G.D. and Torrie, J.H. 1980. Principles and Procedures of Statistics. A Biometrical Approach. 2nd Edition. McGraw-Hill Inc., Toronto.
Stone, K.J. and Townsley, B.H. 1973. The effect of L-ascorbate on catecholamine biosynthesis. Biochem. J. 131: 611–613.
Subramanian, N. 1977. On the brain ascorbic acid and its importance in metabolism of biogenic amines. Life Sci. 20: 1479–1484.
Wilson, J.X. 1987. Ascorbic acid regulates norepinephrine uptake by cultured cerebral astrocytes. Abstract: 30th Ann. Meeting Canadian Federation of Biological Societies, Winnipeg, Canada.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Johnston, W.L., MacDonald, E. & Hilton, J.W. Relationships between dietary ascorbic acid status and deficiency, weight gain and brain neurotransmitter levels in juvenile rainbow trout,Salmo gairdneri . Fish Physiol Biochem 6, 353–365 (1989). https://doi.org/10.1007/BF01875605
Issue Date:
DOI: https://doi.org/10.1007/BF01875605