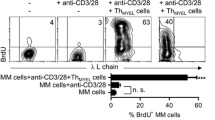
Summary
Short-term cultures containing bone marrow mononuclear cells from multiple myeloma patients secrete monoclonal immunoglobulin and β2-microglobulin into the supernatant, which can be measured quantitatively in an enzyme-linked immunosorbant assay. In this system, the addition of interleukin-2 was shown to induce tumor cell regression in the cultures from 10 out of 14 multiple myeloma patients in a dose-dependent manner. Marker analyses of culture cell populations indicate that OKT3 antibody or interleukin-2 did not directly act on the malignant clone but augmented autologous T lymphocytes, which were responsible for the regression of tumor cells in the cultures.
Similar content being viewed by others
References
Abbas AK (1979) Antigen and T lymphocyte mediated suppression of myeloma cells: Model systems for regulation of lymphocyte function. Immunol Rev 48: 245
Bataille R, Grenier J, Commes T (1988) In vitro production of beta 2 microglobulin by human myeloma cells. Cancer Invest 6: 271
Cresswell P, Springer T, Strominger J, Turner MJ, Grey HH, Kubo RT (1974) Immunological identity of the small sub-unit of the HLA antigens and beta-2-microglobulin and its turnover on the cell membrane. Proc Natl Acad Sci USA 71: 2123
Durie BGM, Salmon SE (1975) A clinical staging system for multiple myeloma. Cancer 36: 842
Fujiwara H, Moriyama Y, Suda T, Tsuchida T, Shearer GM, Hamaoka T (1984) Enhanced TNP-reactive helper T cell activity and its utilization in the induction of amplified tumor immunity that results in tumor regression. J Immunol 132: 1571
Henney CS, Kuribayashi K, Kern DE, Gillis S (1981) Interleukin-2 augments natural killer cell activity. Nature 291: 335
Hercend T, Griffin JD, Bensussan A, Schmidt RE, Edson MA, Brennan A, Murray C, Daley JF, Schlossman SF, Ritz J (1985) Generation of monoclonal antibodies to a human killer clone. Characterization of two natural killer-associated antigens, NKH1 and NKH2 expressed on subsets of large granular lymphocytes. J Clin Invest 75: 932
Izaguirre CA, Minden MD, Howatson AF, McCulloch EA (1980) Colony formation by normal and malignant human B-lymphocytes. Br J Cancer 42: 430
Jackson N, Ling NR, Ball J, Bromidge E, Nathan PD, Franklin IM (1988) An analysis of myeloma plasma cell phenotype using antibodies defined at the IIIrd international workshop on human leucocyte differentiation antigens. Clin Exp Immunol 72: 351
Kabelitz D, Kirchner H, Armerding D, Wagner H (1985) Recombinant interleukin 2 rapidly augments human natural killer cell activity. Cell Immunol 93: 38
Kradin RL, Boyle LA, Preffer FI, Callahan RJ, Barlai-Kovach M, Strauss HW, Dubinett S, Kurnick JT (1987) Tumorderived interleukin-2 dependent lymphocytes in adoptive immunotherapy of lung cancer. Cancer Immunol Immunother 24: 76
Kurnick JT, Kradin RL, Blumberg R, Schneeberger EE, Boyle LA (1986) Functional characterization of T lymphocytes propagated from human lung carcinomas. Clin Immunol Immunopathol 38: 367
Lorbacher P, Yam T, Mitus WJ (1967) Cytochemical demonstration of beta-glucoronidase activity in blood and bone marrow cells. J Histochem Cytochem 15: 680
Lynch RG, Rohrer JW, Odermatt B, Gebel HM, Autry JR, Hoover RG (1979) Immunoregulation of murine myeloma cell growth and differentiation: a monoclonal model for B cell differentiation. Immunol Rev 48: 45
Maekawa R, Matsumoto M, Kitagawa T, Harada M, Sato K (1986) Effect of recombinant interleukin 2 (R-IL2) on in vivo growth of murine myeloma X5563. Cancer Immunol Immunother 23: 25
Mellstedt H, Holm G, Pettersson D, Peest D (1982) Idiotype bearing lymphoid cells in plasma cell neoplasia. In: Salmon SE (ed) Clinics in haematology, vol. 11, no. 1. pp 65–86, Saunders, London, UK
Messner RP (1984) β2-Microglobulin: an old molecule assumes a new look. J Lab Clin Med 104: 141
Meuer SC, Meyer zum Büschenfelde K-H (1986) T cell receptor triggering induces responsiveness to interleukin 1 and interleukin 2 but does not lead to T cell proliferation. J Immunol 136: 4106
Peest D, Holm G, Mellstedt H, Pettersson D (1982) In vitro production of monoclonal and polyclonal Immunoglobulins by peripheral blood mononuclear cells in human plasma cell myeloma. Scand J Immunol 15: 595
Peest D, Bartels B, Dallmann I, Schedel I, Deicher H (1986) Cytostatic drug sensitivity test for human multiple myeloma, measuring monoclonal immunoglobulin produced by bone marrow cells in vitro. Cancer Chemother Pharmacol 17: 69
Peest D, Gasch S, Thiele C, Bartels B, Brunkhorst U, Dallmann I, Hoffmann M, Schedel I, Deicher H (1986) Regulation of the in vitro monoclonal immunoglobulin production in cultures of peripheral blood and bone marrow mononuclear cells from myeloma patients mediated by T cell dependent mitogens. Clin Exp Immunol 65: 120
Reinherz EL, Meuer S, Fitzgerald KA, Hussey RE, Levine H, Schlossman SF (1982) Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell 30: 735
Salmon SE, Wampler SE (1977) Multiple myeloma: quantitative staging and assessment of response with a programmable pocket calculator. Blood 49: 379
Salmon SE, Smith BA (1970) Immunoglobulin synthesis and total body tumor cell number in IgG multiple myeloma. J Clin Invest 49: 1114
Shimazaki C, Atzpodien J, Wisniewski D, Gulati SC, Kolitz JE, Fried J, Clarkson BD (1988) Cell-mediated toxicity of interleukin-2-activated lymphocytes against autologous and allogenic human myeloma cells. Acta Haematol 80: 203
Wagner H, Hardt C, Heeq K, Röllinghoff M, Pfitzenmaier K (1980) T-cell-derived helper factor allows in vivo induction of cytotoxic T cells in nu/nu mice. Nature 284: 278
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Peest, D., de Vries, I., Hölscher, R. et al. Effect of interleukin-2 on the ex vivo growth of human myeloma cells. Cancer Immunol Immunother 30, 227–232 (1989). https://doi.org/10.1007/BF01665009
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01665009