Summary
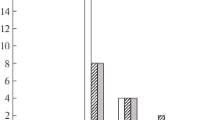
An inverse relationship exists between the sialic acid content of a particle and its ability to activate the alternative complement pathway. The present studies were performed to determine if the neuraminidase (NANase) activities of different mumps virus strains could influence the ability of mumps virus infected cells to activate the alternative pathway. CV-1 cells were infected with three different mumps virus strains (RW, O'Take, and Kilham) and after 24 hours, 10 percent guinea pig serum (GPS) treated with EGTA/MgCl2 or GPS lacking the 4th component of complement (C4DGPS) was added to the cell monolayers. After 30 minutes, the percentage C3 consumed was determined by a functional hemolytic assay. Cells infected with RW (high NANase) consumed significantly more C3 (23.2 per cent) than cells infected with Kilham (5.7 percent, low NANase). Cells infected with O'Take were intermediate in their ability to activate C3. The degree of C3 deposition on the surface of infected cells, detected by fluorescence microscopy, was also greater for cells infected with the RW than the Kilham strain of mumps virus. These studies suggest that the NANase activity of mumps virus can influence the ability of infected cells to activate the alternative pathway and thereby, the ability of complement to participate in host defense against mumps virus infection.
Similar content being viewed by others
References
Ellman, L., Green, I., Frank, M.: Genetically controlled complete deficiency of the fourth component of complement in the guinea pig. Science170, 74–75 (1970).
Fearon, D. T.: Regulation by membrane sialic acid of B1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc. Nat. Acad. Sci. (U.S.A.)75, 1791–1794 (1978).
Hirsch, R. L.: The Complement System: Its importance in the host response to viral infection. Microbiol. Rev.46, 71–85 (1982).
Hirsch, R. L., Winkelstein, J. A., Griffin, D. E.: The role of complement in viral infections. III. Activation of the classical and alternative pathways by Sindbis virus. J. Immunol.124, 2507–2510 (1980).
Hirsch, R. L., Griffin, D. E., Winkelstein, J. A.: Host modification of Sindbis virus sialic acid content influences alternative complement pathway activation and virus clearance. J. Immunol.127, 1740–1743 (1981).
Hirsch, R. L., Griffin, D. E., Winkelstein, J. A.: Natural immunity to Sindbis virus is influenced by host tissue sialic acid content. Proc. Nat. Acad. Sci. (U.S.A.)80, 548–550, (1983).
Kabat, E., Mayer, M. M.: Kabat und Mayer's Experimental Immunochemistry, 2nd ed., 133–240. Springfield, Ill.: Charles C Thomas, 1967.
Kazatchkine, M. D., Fearon, D. T., Austen, K. F.: Human alternative complement pathway: Membrane-associated sialic acid regulates the competition between B and B1H for cell bound C3b. J. Immunol.122, 75–81 (1979).
Lambre, C. R., Kazatchkine, M. D., Maillet, F., Thibon, M.: Guinea pig erythrocytes, after their contact with influenza virus, acquire the ability to activate the human alternative pathway through virus-induced desialation of the cells. J. Immunol.126, 629–634 (1982).
McCarthy, M., Jubelt, B., Fay, D. B., Johnson, R. T.: Comparative studies of five mumps strainsin vitro and in neonatal hamsters: evaluation of growth, cytopathogenecity, and neurovirulence. J. Med. Virol.5, 1–15 (1980).
McSharry, J. J., Pickering, R. J., Caliguiri, L. A.: Activation of the alternative pathway by enveloped viruses containing limited amounts of sialic acid. Virology114, 507–515 (1981).
Merz, D. C., Wolinsky, J. S.: Biochemical features of mumps virus neuraminidases and their relationship with pathogenicity. Virology114, 218–227 (1981).
Oldstone, M. B. A., Lampert, R. N.: Antibody mediated complement dependent lysis of virus infected cells. Springer Seminars in Immunopathology, Vol. 2, 261–283 (1979).
Server, A. C., Merz, D. C., Waxham, M. N., Wolinsky, J. S.: Differentiation of mumps virus strains with monoclonal antibody to the HN glycoprotein. Infect. Immun.35, 179–186 (1982).
Stollar, V., Stollar, B. D., Koo, R., Harrap, K. A., Schlesinger, R. W.: Sialic acid content of Sindbis virus from vertebrate and mosquito cells. Equivalence of biological and immunological viral properties. Virology69, 104–115 (1976).
Warren, L.: The thiobarbituric acid assay of sialic acids. J. Biol. Chem.234, 1971–1975 (1961).
Welsh, R. M.: Host cell modification of lymphocytic choriomeningitis virus and New Castle disease virus altering viral inactivation by human complement. J. Immunol.118, 348–354 (1977).
Author information
Authors and Affiliations
Additional information
With 1 Figure
Rights and permissions
About this article
Cite this article
Hirsch, R.L., Wolinsky, J.S. & Winkelstein, J.A. Activation of the alternative complement pathway by mumps infected cells: Relationship to viral neuraminidase activity. Archives of Virology 87, 181–190 (1986). https://doi.org/10.1007/BF01315298
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01315298