Summary
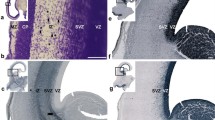
A light and electron microscopic study of the developing corpus callosum was carried out in foetal and neonatal rats in order to determine the mode of growth of the earliest callosal axons across the midline and to investigate the potential role played by non-neuronal cells during the formation of the tract. The axons of the corpus callosum first cross the midline between the 18th and 19th days of gestation by traversing the anterodorsal aspect of the pre-existing hippocampal commissure. Prior to the appearance of the callosal axons at the midline, there is an aggregation of astrocyte processes anterior and dorsal to the hippocampal commissure. Careful examination of these processes in different planes of section shows that they are not organized in any obvious way that would provide a clearly defined path for the growing axons; nor are there any preferentially oriented extracellular spaces at the midline. No specialized membrane contacts could be seen between non-neuronal cell processes and the early callosal axons. Thus, there is no overt morphological evidence for an active role of non-neuronal cells in axon guidance in the initial formation of the corpus callosum.
The development of the corpus callosum is accompanied by the formation of a temporary cavum septi pellucidi, which is always closed to the subarachnoid space. The cavum persists during the first postnatal week, after which time it becomes populated by cells of the lateral septal nuclei. Macrophages are present within the cavum and may play a role in its formation. Macrophages are also found within parts of the corpus callosum. No obvious degeneration of axons is seen in the corpus callosum during its early development.
Similar content being viewed by others
References
Abbie, A. A. (1939) The origin of the corpus callosum and the fate of the structures related to it.Journal of Comparative Neurology 70, 9–44.
Berry, M. &Rogers, A. W. (1965) The migration of neuroblasts in the developing cerebral cortex.Journal of Anatomy 99, 691–709.
Bunge, M. B. (1973) Fine structure of nerve fibers and growth cones of isolated sympathetic neurons in culture.Journal of Cell Biology 56, 713–35.
Dart, R. A. (1925) The genesis of the cavum septi pellucidi.Journal of Anatomy 59, 369–78.
Egar, M. &Singer, M. (1972) The role of ependyma in spinal cord regeneration in the urodele,Triturus.Experimental Neurology 37, 422–30.
Fiori, M. G. &Mugnaini, E. (1981) Microglial-like cells in the chicken ciliary ganglion.Neuroscience 6, 667–77.
Goldby, F. (1940) On the relative position of the hippocampus and the corpus callosum in placental mammals.Journal of Anatomy 74, 227–38.
His, W. (1889) Die Formentwickelung des menschlichen Vorderhirns vom ende des ersten bis zum beginne des dritten Monates.Abhandlungen der mathematisch-physischen Classe der könig. Sächsischen Gesellschraft der Wissenschaften Bd. 15, 675–735.
His, W. (1904)Die Entwickelung des menschlichen Gehirns während der ersten Monate. Leipzig: S. Hirzel.
Hochstetter, F. (1929)Beitrage zur Entwicklungsgeschicte des menschlichen Gehirns. Wien und Leipzig: F. Deuticke.
Imamoto, K. &Leblond, C. P. (1978) Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells.Journal of Comparative Neurology 180, 139–64.
Innocenti, G. M., Fiore, L. &Caminiti, R. (1977) Exuberant projection into the corpus callosum from the visual cortex of newborn cats.Neuroscience Letters 4, 237–42.
Ivy, G. O., Akers, R. M. &Killackey, H. P. (1979) Differential distribution of callosal projection neurons in the neonatal and adult rat.Brain Research 173, 532–7.
Ivy, G. O. &Killackey, H. P. (1981) The ontogeny of the distribution of callosal projection neurons in the rat parietal cortex.Journal of Comparative Neurology 195, 367–89.
Johnston, J. B. (1913) The morphology of the septum, hippocampus, and pallial commissures in reptiles and mammals.Journal of Comparative Neurology 23, 371–478.
Krayanek, S. &Goldberg, S. (1981) Oriented extracellular channels and axonal guidance in the embryonic chick retina.Developmental Biology 84, 41–50.
Langford, L. A. &Coggeshall, R. E. (1980) The use of potassium ferricyanide in neural fixation.Anatomical Record 197, 297–303.
Lavail, J. H. &Lavail, M. M. (1974) The retrograde intraaxonal transport of horseradish peroxidase in the chick visual system: A light and electron microscopic study.Journal of Comparative Neurology 157, 303–57.
Long, J. A. &Burlingame, P. L. (1938) The development of external form of the rat, with some observations on the origin of the extraembryonic coelom and foetal membranes.University of California (Berkeley)Publications in Zoology 43, 143–83.
Michel, M. C. &Reier, P. J. (1979) Axonal-ependymal associations during early regeneration of the transected spinal cord inXenopus laevis tadpoles.Journal of Neurocytology 8, 529–48.
Mihalkovics, V. V. (1877)Enwicklungsgeschichte des Gehirns. Nach Untersuchungen an höheren Wirbeltieren und den Menschen. Leipzig: W. Englemann.
Nordlander, R. H. &Singer, M. (1978) The role of ependyma in regeneration of the spinal cord in the urodele amphibian tail.Journal of Comparative Neurology 180, 349–74.
O'leary, D. D. M., Stanfield, B. B. &Cowan, W. M. (1981) Evidence that the early postnatal restriction of the cells of origin of the callosal projection is due to the elimination of axonal collaterals rather than to the death of neurons.Developmental Brain Research 1, 607–17.
Rakic, P. (1971) Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electron microscopic study inMacacus rhesus.Journal for Comparative Neurology 141, 283–312.
Rakic, P. &Yakovlev, P. I. (1968) Development of the corpus callosum and cavum septi in man.Journal of Comparative Neurology 132, 45–72.
Reynolds, E. S. (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy.Journal of Cell Biology 17, 208–12.
Richardson, K. D., Jarett, L. &Finke, E. H. (1960) Embedding in epoxy resins for ultrathin sectioning in electron microscopy.Stain Technology 35, 313–23.
Schmechel, D. C. &Rakic, P. (1979) A Golgi study of radial glial cells in developing monkey telencephalon: Morphogenesis and transformation into astrocytes.Anatomy and Embryology 156, 115–52.
Schreyer, D. J. &Jones, E. G. (1980) Growth of the corticospinal tract in neonatal rats.Neuroscience Abstracts 6, 649.
Schreyer, D. J. &Jones, E. G. (1981a) Growth of the corticospinal axons through lesioned neonatal spinal cord.Neuroscience Abstracts 7, 549.
Schreyer, D. J. &Jones, E. G. (1981b) Growth and target finding by axons of the corticospinal tract in prenatal and postnatal rats.Neuroscience (in press).
Silver, J. (1980) Mechanisms of axonal guidance during the formation of central nervous system commissures.Neuroscience Abstracts 6, 487.
Silver, J. &Sidman, R. L. (1980) A mechanism for the guidance and topographic patterning of retinal ganglion cell axons.Journal of Comparative Neurology 189, 101–11.
Singer, M., Nordlander, R. H. &Egar, M. (1979) Axonal guidance during embryogenesis and regeneration in the spinal cord of the newt: The blueprint hypothesis of neuronal pathway patterning.Journal of Comparative Neurology 185, 1–22.
Smith, G. E. (1897) The origin of the corpus callosum: A comparative study of the hippocampal region of the cerebrum of marsupialia and certain cheiroptera.Transactions of the Linnean Society of London, Second Series, Zoology 7, 47–69.
Smith, G. E. (1903) Zuckerkandl on the phylogeny of the corpus callosum.Anatomischer Anzeiger 23, 384–90.
Smith, G. E. (1910) The Arris and Gale lectures on some problems relating to the evolution of the brain.Lancet, 1–6, 147–55, 221–27.
Sturrock, R. R. (1976) Light microscopic identification of immature glial cells in semithin sections of the developing mouse corpus callosum.Journal of Anatomy 122, 521–37.
Tennyson, V. (1970) The fine structure of the axon and growth cone of the dorsal root neuroblast of the rabbit embryo.Journal of Cell Biology 44, 62–79.
Valentino, K. L. &Jones, E. G. (1980) An electron microscopic study of the developing corpus callosum in foetal and neonatal rats.Neuroscience Abstracts 6, 487.
Valentino, K. L. &Jones, E. G. (1981) Morphological and immunocytochemical identification of macrophages in the developing corpus callosum.Anatomy and Embryology 163, 157–72.
Vaughn, J. E. &Peters, A. (1968) A third neuroglial cell type. An electron microscopic study.Journal of Comparative Neurology 133, 267–88.
Vaughn, J. E., Hinds, P. L. &Skoff, R. P. (1970) Electron microscopic studies of Wallerian degeneration in rat optic nerves. I. The multipotential glia.Journal of Comparative Neurology 140, 175–206.
Wahlsten, D. (1981) Prenatal schedule of appearance of mouse brain commissures.Developmental Brain Research 1, 461–74.
Watson, J. L. (1958) Staining of tissue sections for electron microscopy with heavy metals.Journal of Biophysical and Biochemical Cytology 4, 475–8.
Wise, S. P. &Jones, E. G. (1976) The organization and postnatal development of the commissural projection of the rat somatic sensory cortex.Journal of Comparative Neurology 168, 313–44.
Zuckerkandl, E. (1901) Zur Entwicklung des Balkens und des Gewölbes.Sitzungsberichte der kaiserlich Akademie der Wissenschaften, Wien, mathematisch-naturwissenschaftliche Classe. Bd. 110, Abt.3, 233–307.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Valentino, K.L., Jones, E.G. The early formation of the corpus callosum: a light and electron microscopic study in foetal and neonatal rats. J Neurocytol 11, 583–609 (1982). https://doi.org/10.1007/BF01262426
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01262426