Abstract
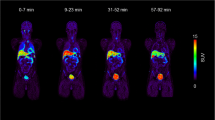
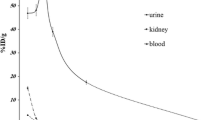
The synthetic amino acidl-3-[123I]iodo-α-methyltyrosine (IMT) is currently under clinical evaluation as a single-photon emission tomography (SPET) tracer of amino acid uptake in brain tumours. So far, dosimetric data in respect of IMT are not available. Therefore we investigated the whole-body distribution of IMT in six patients with cerebral gliomas and the radiation doses were estimated. Whole-body scans were acquired at 1.5, 3 and 5 h after i.v. injection of 370–550 MBq IMT. The bladder was voided prior to each scan and the radioactivity excreted in the urine was measured. Based on the MIRD-11 method and the updated MIRDOSE3, the mean absorbed doses for various organs and the effective dose were calculated from geometric means of the anterior and posterior whole-body scans using seven source organs and the residence time. IMT was predominantly excreted by the kidneys (52.8%±11.5% at 1.5 h p.i., 63.0%±15.7% at 3 h p.i. and 74.6%±9.8% at 5 h p.i.). No organ system other than the urinary tract showed significant retention of the tracer. Early whole-body scans revealed slightly increased tracer uptake in the liver and in the bowel. Highest absorbed doses were found for the urinary bladder wall (0.047 mGy/MBq), the kidneys (0.010 mGy/MBq), the lower large intestinal wall (0.011 mGy/MBq) and the upper large intestinal wall (0.008 mGy/MBq). The effective dose according to ICRP 60 was estimated to be 0.0073 mSv/MBq for adults. This leads to an effective dose of 3.65 mSv in a typical brain SPET study using 500 MBq IMT. The MIRDOSE3 scheme yielded similar results. Thus, in spite of the relatively high tracer dose required for optimal brain scanning, radiation exposure in SPET studies with IMT is in the normal range of routine nuclear medicine investigations.
Similar content being viewed by others
References
Derlon JM, Bourdet C, Bustany P, Chatel M, Theron J, Darcel F, Syrota A. [11C]-l-methionine uptake in gliomas.Neurosurgery 1989; 25: 720–728.
Ogawa T, Shishido F, Kanno I, Inugami A, Fujita H, Murakami M, Shimosegawa E, Ito H, Hatazawa J, Okudera T, Uemura K, Yasui N Mineura K. Cerebral glioma: evaluation with methionine PET.Radiology 1993; 186: 45–53.
Mosskin M, Ericson K, Hindmarsh T, von Holst H, Collins VP, Bergstrom M, Eriksson L, Johnstrom P. Positron emission tomography compared with magnetic resonance imaging and computed tomography in supratentorial gliomas using multiple stereotactic biopsies as reference.Acta Radiol 1989; 30: 225–232.
Biersack HJ, Coenen HH, Stöcklin G, Reichmann K, Bockisch A, Oehr P, Kashab M, Rollmann O. Imaging of brain tumors withl-3-[123I]Iodo-α-methyl tyrosine and SPECT.J Nucl Med 1989; 30: 110–112.
Langen KJ, Coenen HH, Roosen N, Kling P, Muzik O, Herzog H, Kuwert T, Stöcklin G, Feinendegen LE. SPECT studies of brain tumors withl-3-[123I]Iodo-α-methyl tyrosine: comparison with PET,124IMT and first clinical results.J Nucl Med 1990; 31: 281–286.
Langen KJ, Roosen N, Coenen HH, Kuikka JT, Kuwert T, Herzog H, Stöcklin G, Feinendegen LE. Brain and brain tumor uptake ofl-3-[123I]Iodo-α-methyl tyrosine: competition with naturall-amino acids.J Nucl Med 1991; 32:1225–1228.
Kawai K, Fujibayashi Y, Saji H, Yonekura Y, Konishi J, Kubodera A, Yokoyama A. A strategy for the study of cerebral amino acid transport using iodine-123-labelled amino acid radiopharmaceutical: 3-iodo-alpha-methyl-l-tyrosine.J Nucl Med 1991; 32: 819–824.
Wienhard K, Herholz K, Coenen HH, Rudolf J, Kling P, Stöcklin G, Heiss WD. Increased amino acid transport into brain tumors measured by PET ofl-[2-18F]fluorotyrosine.J Nucl Med 1991; 32: 1338–1346.
Langen KJ, Ziemons K, Kiwit JCW, Herzog H, Kuwert T, Bock WJ, Stöcklin G, Feinendegen LE, Müller-Gärtner HW. [123I]-Iodo-α-methyl-tyrosine SPELT and [methyl-11C]-l-me-thionine uptake in cerebral gliomas: a comparative study using SPECT and PET.J Nucl Med 1997; 38: 517–522.
Kuwert T, Morgenroth C, Woesler B, Matheja P, Palkovic S, Vollet B, Samnick S, Maasjosthusmann U, Lerch H, Gildehaus FJ, Wassmann H, Schober O. Uptake of iodine-123-α-methyl tyrosine by gliomas and non-neoplastic brain lesions.Eur J Nucl Med 1996; 23: 1345–1353.
Guth-Tougelidis B, Müller S, Mehdom MM, Knust EJ, Dutschka K, Reiners C. Anreicherung vondl-3-123I-α-methyltyrosin in Hirntumorrezidiven.Nucl-Med 1995: 34: 71–75.
Schmidt D, Wunderlich G, Langen KJ, Ziemons K, Kiwit JCW, Holschbach M, Müller-Gärtner HW I-123-α-methyl-tyrosine (IMT) SPELT for evaluation of chemotherapy in cerebral gliomas.J Nucl Med 1996; 37: 354P.
Tisljar U, Kloster G, Ritzl F, Stöcklin G. Accumulation of radioiodinatedl-α-methyltyrosine in pancreas of mice: concise communication.J Nucl Med 1979: 20: 973–976.
Kloster G, Bockslaff H.l-3-123I-α-methyltyrosine for melanoma detection: a comparative evaluation.Int J Nucl Med Biol 1982; 9: 259–269.
Krummeich C, Holschbach M, Stöcklin G. Direct electrophilic radioiodinadon of tyrosine analogues: their in-vivo stability and brain uptake in mice.Appl Radiat Isot 1994; 45: 929–935.
Budinger TF. Quantitative nuclear medicine imaging: application of computers to the gamma camera and whole-body scanner. In:Recent Advances in Nuclear Medicine 1974: 41–130.
Sorensen JA. Quantitative measurement of radioactivity in vivo by whole-body counting. In: Hine GJ, et al., eds.Instrumentation in nuclear medicine, vol 2. New York: Academic Press; 1974: 311–347.
Loevinger R, Budinger TF, Watson EE.MIRD — primer for absorbed dose calculations. New York: The Society of Nuclear Medicine, 1988.
Snyder WS, Ford MR, Warner GG, Watson SB. “S,” absorbed dose per unit cumulated activity for selected radionuclides and organs.MIRD Pamphlet No. 11. New York, The Society of Nuclear Medicine, 1975.
Recommendations of the International Commission on Radiological Protection.ICRP publication 60. New York: Pergamon Press, 1991.
Stabin MG. MIRDOSE: personal computer software for internal dose assessment in nuclear medicine.J Nucl Med 1996; 37:538–546.
Deehan B, Carnochan P, Trivedi M, Tombs A. Uptake and distribution ofl-3-[125I]iodo-α-methyl tyrosine in experimental rat tumours: comparison with blood flow and growth rate.Eur J Nucl Med 1993; 20:101–106.
Dey HM, Seibyl JP, Stubbs JB, Zoghbi SS, Baldwin RM, Smith EO, Zubal IG, Zea-Ponce Y, Olson C, Charney DS, Hoffer PB, Innis RB. Human biodistribution and dosimetry of the SPECT benzodiazepie receptor radioligand iodine-123-iomazenil.J Nucl Med 1994; 35: 399–404.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmidt, D., Langen, KJ., Herzog, H. et al. Whole-body kinetics and dosimetry ofl-3-[123I]iodo-α-methyltyrosine. Eur J Nucl Med 24, 1162–1166 (1997). https://doi.org/10.1007/BF01254250
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01254250