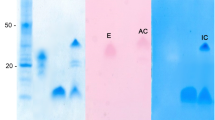
Abstract
The stabilities of trypsin and soybean trypsin inhibitor in sodium dodecylsulfate (SDS) were examined by SDS-polyacrylamide gel electrophoresis (PAGE). Both samples contained several bands, all of which migrated to positions corresponding to the appropriate molecular weight or less, even when the samples were unheated, suggesting that both the trypsin and trypsin inhibitor are susceptible to SDS-induced denaturation. When they were mixed together prior to addition of SDS-PAGE sample buffer (1% SDS), a new smearing band appeared which corresponded to a molecular weight of around 46,000, suggesting that these proteins form a stable complex in SDS. This was confirmed by electroblotting and sequence analysis, which indicated that this band contains both the trypsin and inhibitor sequences. At a fixed concentration of the inhibitor, increasing concentrations of the trypsin resulted in an increase in the intensity of the complex band. When the mixture was heated for 10 min in 1% SDS, the complex band disappeared in a temperature-dependent manner. The melting temperature determined under the experimental conditions used was about 35|MoC. Similar results were obtained with Bowman-Birk trypsin inhibitor, except that the complex with the above inhibitor had a higher melting temperature, around 41|MoC, suggesting that the Bowman-Birk inhibitor/trypsin complex is more stable than the soybean inhibitor/trypsin complex.
Similar content being viewed by others
References
Arakawa, T., Hung, L., and Narhi, L. O. (1991a).J. Protein Chem. (submitted).
Arakawa, T., Lazenby, K., Kolvenbach, C. G., Horan, T. P., McGinley, M., Rohde, M. F., and Narhi, L. O. (1991b).Agric. Biol. Chem. 55, 903–910.
Horan, T. P., and Arakawa, P. (1990).Agric. Biol. Chem. 54, 563–565.
Kolvenbach, C. G., Narhi, L. O., Lazenby, K., Samal, B., and Arakawa, T. (1990).Int. J. Peptide Protein Res. 36, 387–391.
Laemmli, U. K. (1970).Nature 227, 680–685.
Matsudaira, P. (1987).J. Biol. Chem. 262, 10,035–10,038.
Narhi, L. O., and Arakawa, T. (1989).Biochim. Biophys. Acta 990, 144–149.
Narhi, L. O., Connor, J., Rohde, M. F., McGinley, M. G., and Arakawa, T. (1991).J. Protein Chem. (in press).
Narhi, L. O., Stabinski, Y., Levitt, M., Miller, L., Sachdev, R., Finley, S., Park, S., Kolvenbach, C., Arakawa, T., and Zukowski, M. (1991).Biotech. Appl. Biochem. 13, 12–24.
Narhi, L. O., Zukowski, M., and Arakawa, T. (1988).Arch. Biochem. Biophys. 261, 161–169.
Sweadner, K. G. (1991).Anal. Biochem. 194, 680–685.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arakawa, T., Hung, L., McGinley, M.G. et al. Induced resistance of trypsin to sodium dodecylsulfate upon complex formation with trypsin inhibitor. J Protein Chem 11, 171–176 (1992). https://doi.org/10.1007/BF01025222
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01025222