Abstract
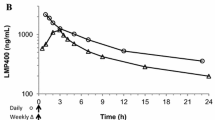
Topotecan is a novel semisynthetic derivative of the anticancer agent camptothecin and inhibits the intranuclear enzyme topoisomerase I. The lactone structure of topotecan, which is in equilibrium with the inactive ringopened hydroxy acid, is essential for this activity. The open form predominates at physiological pH. We performed a pharmacokinetic, study as part of a phase I study in patients with various types of solid tumors, where topotecan was administered in a 30-min infusion daily on 5 consecutive days every 3 weeks. The plasma kinetics of topotecan could be described best using an open two-compartment model with t1/2(α) and t1/2(β) of 8.1 (range 0.3 to 40.7) min and 132 (range 49 to 286) min, respectively. The plasma concentration-time profiles of the metabolite, however, could be described using a one-compartment model with t1/2(formation) of 29.0 (range 5.6–99.5) min and t1/2 (elimination of 123.2 (range 32–265) min, respectively. The lactone was the predominate form during the first hour from the start of infusion, but was rapidly converted into its ring-opened structure. The elimination rate of topotecan was independent of the dose. There were linear relationships between the dose (mg m−2 day−1), the area under the plasma concentration versus time curve (AUC) of topotecan and its metabolite, the total AUC, peak plasma lactone concentrations, and the time period that the topotecan concentrations remained above 10 nM. Different models were used to correlate pharmacokinetic and pharmacodynamic parameters. The percentage decrease in absolute neutrophil count (ANC) was related to these parameters and plots were well fitted by linear and sigmoidal Emax models.
Similar content being viewed by others
References
Anonymous (1988) DNA topoisomerases — new twists to tumour therapy. Lancet I: 512
Beijnen JH, Smith BR, Keijer WJ, Van Gijn R, Ten Bokkel Huinink WW, Vlasveld LT, Rodenhuis S, Underberg WJM (1990) Highperformance liquid chromatographic analysis of the new antitumour drug SK&F 104864-A (NSC 609699) in plasma. J Pharm Biomed Anal 8: 789
Blaney SM, Balis FM, Cole DE, Craig C, Reid JM, Ames MM, Krailo M, Reaman G, Hammond D, Poplack DG (1993) Pediatric phase I trial and pharmacokinetic study of topotecan administered as a 24-hour continuous infusion. Cancer Res 53: 1032
Burris H, Kuhn J, Johnson R, Von Hoff D (1990) Preclinical studies of a new topoisomerase I inhibitor. Proc Am Assoc Clin Oncol 31: 431
Burris HA, Rothenberg ML, Kuhn JG, Von Hoff DD (1992) Clinical trials with the topoisomerase I inhibitiors. Semin Oncol 19: 663
Eckhart J, Burris H, Kuhn J, Smith S, Rodriguez G, Weiss G, Smith L, Shaffer D, Johnson R, Von Hoff D (1992) Phase I and pharmacokinetic trial of continuous infusion of topotecan in patients with refractory solid tumors. Proc Am Soc Clin Oncol 11: 138
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Dekker, New York Basel
Gottlieb JA, Guarino AM, Call JB, Oliverio VT, Block JB (1970) Preliminary pharmacologic and clinical evaluation of camptothecin sodium. Cancer Chemother Rep 54: 461
Grochow LB, Rowinsky EK, Johnson R, Ludeman S, Kaufmann SH, McCabe FL, Smith BR, Hurowitz L, DeLisa A, Donehower RC, Noe D (1992) Pharmacokinetics and pharmacodynamics of topotecan in patients with advanced cancer. Drug Metab Dispos 20: 706
Hertzberg RP, Caranfa MJ, Hecht SM (1989) On the mechanism of topoisomerase I inhibition by camptothecin: evidence for binding to an enzyme-DNA complex. Biochemistry 32: 715
Hertzberg RP, Caranfa MJ, Holden KG, Jakas DR, Gallagher G, Mattern MR, Mong SM, Bartus JO, Johnson RK, Kingsbury WD (1989) Modification of the hydroxy lactone ring of camptothecin: inhibition of mammalian topoisomerase I and biological activity. J Med Chem 32: 715
Houghton PJ, Cheshire PJ, Myers L, Stewart CF, Synold TW, Houghtom JA (1992) Evaluation of 9-dimethylaminomethyl-10-hydroxycamptothecin against xenografts derived from adult and childhood solid tumors. Cancer Chemother Pharmacol 31: 229
Hsiang YH, Lihou MG, Liu LF (1989) Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res 49: 5077
Investigators brochure (1990) Topotecan (SK&F 104864-A). SmithKline Beecham Pharmaceuticals, Philadelphia, USA
Jodrell DI, Egorin MJ, Canetta RM, Langenberg P, Goldbloom EP, Burroughs JN, Goodlow JL, Tan S, Wiltshaw E (1992) Relationships between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. J Clin Oncol 10: 520
Kantarjian HM, Beran M, Ellis A, Zwelling L, O'Brien S, Cazenave L, Koller C, Rios MB, Plunkett W, Keating M, Estey EH (1993) Phase I study of topotecan, a new topoisomerase I inhibitor, in patients with refractory or relapsed acute leukemia. Blood 81: 1146
Kudelka A, Edwards C, Freedman R, Wallin B, Hord M, Howell E, Harper K, Raber M, Kavanaugh J (1993) A phase II study of topotecan administered intravenously as 5 daily infusions every 21 days to women with refractory epithelial ovarian carcinoma. Eur J Cancer 29A [Suppl 6: S132
Moertel CG, Schutt AJ, Reitemeier RJ, Hahn RG (1972) Phase II study of camptothecin in the treatment of advanced gastrointestinal cancer. Cancer Chemother Rep 56: 95
Muggia FM, Creaven PJ, Hansen H, Cohen MH, Selawry OS (1972) Phase I clinical trial of weekly and daily treatment with camptothecin: correlation with preclinical studies. Cancer Chemother Rep 56: 515
Perez-Soler R, Glisson BS, Kane J, Raber MN, Hong WK (1993) Phase II study of topotecan in patients with non-small cell lung cancer. Eur J Cancer 29A [Suppl 6]: S162
Proost JH, Meijer DKF (1992) MW/PHARM, An integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med 22: 155
Rowinsky EK, Grochow LB, Hendriks CB, Ettinger DS, Forastiere AA, Hurowitz LA, McGuire WP, Sartorius SE, Lubejko BG, Kaufmann SH, Donehower RC (1992) Phase I and pharmacologic study of topotecan: a novel topoisomerase I inhibitor. J Clin Oncol 10: 647
Saltz L, Sirott M, Young C, Tong W, Niedzwiecki D, Tzy-Jyun Y, Tao Y, Trochanowski B, Wright P, Barbosa K, Toomasi F, Kelsen D (1993) Phase I clinical and pharmacology study of topotecan given daily for 5 consecutive days to patients with advanced solid tumors, with attempt at dose intensification using recombinant granulocyte colony-stimulating factor. J Natl Cancer Inst 18: 1499
Scher R, Lush CJ, Green K, Kosierowski R, Simmonds M, Engstrom PF, O'Dwyer PJ (1993) Phase II trial of topotecan in advanced pancreatic cancer. Eur J Cancer 29A [Suppl 6]: S101
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9: 503
Sirott MN, Saltz L, Young C, Tong W, Trochanowski B, Niedzwiecki D, Toomasi F, Kelsen D (1991) Phase I and clinical pharmacologic study of intravenous topotecan. Proc Am Assoc Clin Oncol 10: 104
Slichenmyer WJ, Rowinsky EK, Donehower, RC, Kaufmann SH (1993) The current status of camptothecin analogues as antitumor agents. J Natl Cancer Inst 85: 271
Underberg WJM, Goossen RMJ, Smith BR, Beijnen JH (1990) Equilibrium kinetics of the new experimental anti-tumour compound SK&F 104864-A in aquous solution. J Pharm Biomed Anal 8: 681
Van Warmerdam LJC, Verweij J, Rosing H, Schellens JHM, Maes RAA, Beijnen JH (1994) Limited sampling models for topotecan pharmacokinetics. Ann Oncol 5: 259
Verweij J, Lund B, Beijnen J, Planting A, Boer-Dennert M de, Koier I, Rosing H, Hansen H (1993) Phase I and pharmacokinetic study of topotecan, a new topoisomerase I inhibitor. Ann Oncol 4: 673
Wall ME, Wani MC (1977) Antineoplastic agents from plants. Annu Rev Pharmacol Toxicol 17: 117
Wall J, Burris H, Rodriguez G, Brown T, Weiss G, Kuhn J, Brown J, Johnson R, Friedman C, Mann W, Von Hoff D (1991) Phase I trial of topotecan (SK&F 104864) in patients with refractory solid tumors. Proc Am Assoc Clin Oncol 10: 98
Wall JG, Burris HA, Von Hoff DD, Rodriguez G, Kneuper-Hall R, Shaffer D, O'Rourke T, Brown T, Weiss G, Clark G, McVea S, Brown J, Johnson R, Friedman C, Smith B, Mann WS, Kuhn J (1992) A phase I clinical and pharmacokinetic study of the topoisomerase I inhibitor topotecan (SK&F 104864) given as an intravenous bolus every 21 days. Anticancer Drugs 3: 337
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Warmerdam, L.J.C., Verweij, J., Schellens, J.H.M. et al. Pharmacokinetics and pharmacodynamics of topotecan administered daily for 5 days every 3 weeks. Cancer Chemother. Pharmacol. 35, 237–245 (1995). https://doi.org/10.1007/BF00686554
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686554