Abstract
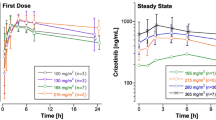
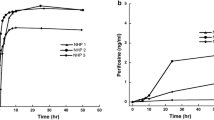
The pharmacokinetics of high-dose fotemustine followed by autologous bone-marrow transplantation during a phase I–II clinical trial in 24 patients with glioblastoma or astrocytoma (grade III–IV) was investigated. Plasma levels of fotemustine were determined by high-performance liquid chromatography (HPLC) and UV detection. The metabolite, 2-chloroethanol, was simultaneously followed in six patients by gag liquid chromatography and electron capture detection (GLC-ECD) assay. The drug was given as a 1-h infusion on 2 consecutive days. In all, 40 pharmacokinetic determinations of fotemustine were made at dose levels ranging from 2×300 to 2×500 mg/m2. Plasma drug elimination was best described by a bi-exponential model, with short distribution and elimination halflives of 4.15±2.57 and 28.8±12.1 min being observed, respectively. No significant difference in half-lives or clearance was seen between the first and the second administration. During dose escalation, the mean area under the concentrationtime curve (AUC) increased from 5.96±2.89 to 12.22±3.95 mg l−1h. Drug clearance was independent of the dose given and equal to 109±65 l/h, indicating no possible saturation of metabolism and elimination mechanisms at these high-dose levels. The metabolite 2-chloroethanol appeared very early in plasma samples. Its elimination was rapid and rate-limited by the kinetics of the parent compound, giving the same apparent terminal half-life. A close relationship between AUC and C45 values was evidenced (r=0.890). Associated with the stability of fotemustine kinetic parameters, this could be used in future studies for individual dose adjustment, particularly for high-dose fractionated regimens.
Similar content being viewed by others
References
Catalin J, Biao-Yin M, Deloffre P, Rustum Y, Tapiero H (1988) Cytotoxic and cytogenetic effects of a new amino acid-linked nitrosourea (S10036). Proceedings, 6th, Congresso Mediterraneao di Chemotherapia, Taormina
Deloffre P, Cudennec CA, Lavielle G, Bizzari JP (1987) Approach of the mechanism of action and mutagenicity of the new nitrosourea Servier 10036. Proceedings, 15th International Congress of Chemotherapy, Istanbul
Gomeni C, Gomeni R (1987) SIPHAR, an integrated computer system for statistical and pharmacokinetic data analysis. In: Serio A, O'Moore R, Tardini A, Roger F (eds) Proceedings of the 7th International Congress of Medical Informatics. European Federation for Medical Informatics. Rome, p 507–516
Gordon BH, Richards RP, Hiley MP, Gray AJ, Ings RMJ, Campbell DB (1989) A new method for the measurement of nitrosoureas in plasma: an H. P. L. C. procedure for the measurement of fotemustine kinetics. Xenobiotica 19: 329–339
Ings RMJ, Gray AJ, Taylor AR, Gordon BH, Breen M, Hiley M, Brownsill R, Marchant N, Richards R, Wallace D, Hughes T, Thomas R, Williams J, Lucas C, Campbell DB (1990) Disposition, pharmacokinetics, and metabolism of14C-fotemustine in cancer patients. Eur J Cancer 26: 838–842
Jacquillat C, Khayat D, Banzet P, Weil M, Fumoleau P, Avril MF, Namer P, Lauvin R, Vilmer C, Prache C, Bizzari JP (1990) Final report on the French multicenter phase II study of the nitrosourea fotemustine in 153 evaluable patients with disseminated malignant melanoma including patients with cerebral metastases. Cancer 66: 1873–1878
Khayat D, Lokiec F, Bizzari JP, Weil M, Meeus L, Sellami M, Rouesse J, Banzet P, Jacquillat C (1987) Phase I clinical study of the new amino acid-linked nitrosourea, S10036, administered on a weekly schedule. Cancer Res 47: 6782–6785
Lemoine A, Lucas C, Ings RMJ (1991) Metabolism of chloroethyl-nitrosoureas: review. Xenobiotica 21: 775–791
Lokiec F, Beerblock K, Deloffre P, Lucas C, Bizzari JP (1989) Etude de pharmacocinétique clinique de la fotémustine dans différentes indications tumorales. Bull Cancer (Paris) 76: 1063–1069
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tranchand, B., Lucas, C., Biron, P. et al. Phase I pharmacokinetics study of high-dose fotemustine and its metabolite 2-chloroethanol in patients with high-grade gliomas. Cancer Chemother. Pharmacol. 32, 46–52 (1993). https://doi.org/10.1007/BF00685875
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685875