Summary
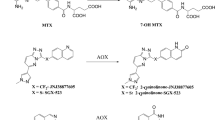
The inhibition of methotrexate (MTX) biotransformation to 7-hydroxymethotrexate (7-OH-MTX) by 4′-(9-acridinylamino)-methanesulfon-m-anisidide (mAMSA) was studied in bile-drained rats in vivo and in incubates of isolated rat hepatocytes and rat-liver homogenate in vitro. In vivo, i.v. administration of 10 mg/kg mAMSA prior to [3H]-MTX infusion (50 mg/kg) led to a significant alteration in 7-OH-MTX kinetics. 7-OH-MTX peak concentrations and AUC in bile and serum were reduced by 75% and the recovery of MTX as 7-OH-MTX in bile and urine decreased by 70%, whereas MTX pharmacokinetics remained unaltered. In suspensions of isolated hepatocytes. 10 μm mAMSA led to a 54% decrease in 7-OH-MTX formation. However, the hepatocellular influx and efflux of MTX was not perturbed by mAMSA. Preincubation of rat-liver homogenates with 1.25–10 μm mAMSA reduced the formation of 7-OH-MTX by up to 73%. mAMSA appeared to inhibit MTX hydroxylation competitively, exhibiting aK iof 3 μm. Due to its inhibition of the MTX-oxidizing system, mAMSA may be beneficial in combination chemotherapy with MTX by reducing 7-OH-MTX-associated toxicity and, possibly, enhancing the cytotoxic effects of MTX.
Similar content being viewed by others
References
Aarbakke J, Ueland PM (1981) Interaction ofS-adenocyl-homocysteine with isolated rat hepatocytes. Mol Pharmacol 19:463
Banerjee AK, Lakhani S, Vincent M, Selby P (1988) Dose-dependent acute hepatitis associated with administration of high dose methotrexate. Hum Toxicol 7:561
Berg T, Mørland J (1975) Induction of tryptophan oxygenase by dexamethasone in isolated hepatocytes. Dependence on composition of medium and pH. Biochim Biophys Acta 392:233
Berry MN, Friend DS (1969) High-yield preparation of isolated rat liver parenchymal cells. J Cell Biol 43:506
Borsi JD, Sagen E, Romslo I, Moe PJ (1990) Comparative study of the pharmacokinetics of 7-hydroxymethotrexate after administration of methotrexate in the dose range of 0.5–33.6 g/m2 to children with acute lymphoblastic leukemia. Med Pediatr Oncol 18:217
Bradford MM (1976) Protein assay by dye binding. Anal Biochem 72:248
Breithaupt H, Kuenzlen E (1982) Pharmacokinetics of methotrexate and 7-hydroxymethotrexate following infusions of high-dose methotrexate. Cancer Treat Rep 66:1733
Breithaupt H, Kuenzlen E (1983) High-dose methotrexate for osteosarcoma: toxicity and clinical results. Oncology 40:85
Bremnes RM, Slørdal L, Wist E, Aarbakke J (1989) Formation and elimination of 7-hydroxymethotrexate in the rat in vivo after methotrexate administration. Cancer Res 49:2460
Bremnes RM, Slørdal L, Wist E, Aarbakke J (1989) Dose-dependent pharmacokinetics of methotrexate and 7-hydroxymethotrexate in the rat in vivo. Cancer Res 49:6359
Bremnes RM, Smeland E, Huseby N-E, Eide TE, Aarbakke J (1991) Acute hepatotoxicity after high-dose methotrexate administration to rats. Pharmacol Toxicol (in press)
Bremnes RM, Smeland E, Slørdal L, Wist E, Aarbakke J (1991) The effect of vindesine on methotrexate disposition in the rat. Biochem Pharmacol (in press)
Cassileth PA, Gale RP (1986) mAMSA: a review. Leukemia Res 10: 1257
Chauvet M, Bourdeaux M, Briand C, Dell'Amico M, Gilli R, Diarra M (1983) Interactions of methotrexate metabolites with beef liver dihydrofolate reductase: I. Biochem Pharmacol 32:1059
Chen M-L, Chiou WL (1982) Tissue metabolism and distribution of methotrexate in rabbits. Drug Metab Dispos 10:706
Coleman R (1987) Biochemistry of bile secretion. Biochem J 244: 249
Cysyk RL, Shoemaker D, Adamson RH (1977) The pharmacologic disposition of 4′-(9-acridinylamino)methanesulfon-m-anisidide in mice and rats. Drug Metab Dispos 5:579
Drake JC, Allegra CJ, Baram J, Kaufman BT, Chabner BA (1987) Effects on dihydrofolate reductase of methotrexate metabolites and intracellular folates formed following methotrexate exposure of human breast cancer cells. Biochem Pharmacol 36:2416
Dukes HH (1947) The exchange of matter and energy. In: The physiology of domestic animals. Comstock, New York, p 415
El-Yazigi A, Amer M, Al-Saleh I, Martin C (1986) Pharmacokineties of methotrexate and its 7-OH metabolite in cancer patients treated with different high-methotrexate dosage regimens. Int J Cancer 38:795
Erttmann R, Bielack S, Landbeck G (1985) Kinetics of 7-hydroxymethotrexate after high-dose methotrexate therapy. Cancer Chemother Pharmacol 15:101
Fabre G, Matherly LH, Favre R, Catalin J, Cano JP (1983) In vitro formation of polyglutamyl derivatives of methotrexate and 7-hydroxymethotrexate in human lymphoblastic leukemia cells. Cancer Res 43:4648
Fabre G, Matherly LH, Fabre I, Cano J-P, Goldman ID (1984) Interactions between 7-hydroxymethotrexate and methotrexate at the cellular level in the Ehrlich ascites tumor in vitro. Cancer Res 44:970
Fabre G, Fabre I, Matherly LH, Cano J-P, Goldman ID (1984) Synthesis and properties of 7-hydroxymethotrexate polyglutamyl derivatives in Ehrlich ascites tumor cells in vitro. J Biol Chem 259: 5066
Farquhar D, Loo TL, Vadlamudi S (1972) Synthesis and biological evaluation of 7-hydroxymethotrexate, 7-methylaminopterin, and 7-methylmethotrexate. J Med Chem 15:567
Fry JR (1981) Preparation of mammalian hepatocytes. Methods Enzymol 77:130
Gaukroger JM, Wilson L (1984) Protection of cells from methotrexate toxicity by 7-hydroxymethotrexate. Br J Cancer 50:327
Gilli RM, Sari JC, Sica LM, Briand CM (1988) Thermodynamic study of the influence of NADPH on the binding of methotrexate and its metabolites to a mammalian dihydrofolate reductase. Biochim Biophys Acta 964:53
Gormley PE, Rossitch E, D'Anna ME, Cysyk R (1983) An extremely potent anilinoacridine inhibitor of aldehyde oxidase. Biochem Biophys Res Commun 116:759
Jacobs SA, Stoller RG, Chabner BA, Johns DG (1976) 7-Hydroxymethotrexate as a urinary metabolite in human subjects and rhesus monkeys receiving high dose methotrexate. J Clin Invest 57:534
Jacobs SA, Stoller RG, Chabner BA, Johns DG (1977) Dose-dependent metabolism of methotrexate in man and rhesus monkeys. Cancer Treat Rep 61:651
Johns DG, Valerino DM (1971) Metabolism of folate antagonists. Ann NY Acad Sci 186:378
Johns DG, Iannotti AT, Sartorelli AC, Booth BA, Bertino JR (1965) The identity of rabbit-liver methotrexate oxidase. Biochim Biophys Acta 105:380
Johns DG, Iannotti AT, Sartorelli AC, Bertino JR (1966) The relative toxicities of methotrexate and aminopterin. Biochem Pharmacol 15:555
Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA (1983) The pharmacology and clinical use of methotrexate. N Engl J Med 309:1094
Jurgens H, Ebell W, Bachmann R (1983) Essential laboratory determinations for monitoring high-dose methotrexate treatment with citrovorum factor rescue. Pediatr Pharmacol 3:157
Klaassen CD, Watkins JB (1984) Mechanisms of bile formation, hepatic uptake, and biliary excretion. Pharmacol Rev 36:1
Lankelma J, Van der Klein E, Ramaekers F (1980) The role of 7-hydroxymethotrexate during methotrexate anticancer chemotherapy. Cancer Lett 9:133
Lazarus HM, Berger NA (1988) Chemotherapy of malignant disease: an update. Compr Ther 14:56
Lee I-J, Chan KK (1988) Metabolic interaction between methotrexate and 4′-(9-acridinylamino)methanesulfon-m-anisidide in the rabbit. Cancer Res 48:5106
Louie AC, Issell BF (1985) MAMSA (AMSA) — a clinical review. J Clin Oncol 3:562
Matherly LH, Seither RL, Goldman ID (1987) Metabolism of the diaminofolates: biosynthesis and pharmacology of the 7-hydroxyl and polyglutamyl metabolites of methotrexate and related antifolates. Pharmacol Ther 35:27
McGuire JJ, Hsieh P, Bertino JR (1984) Enzymatic synthesis of polyglutamate derivatives of 7-hydroxymethotrexate. Biochem Pharmacol 33:1355
Moran RG, Colman PD, Rosowsky A, Forsch RA, Chan KK (1985) Structural features of 4-amino antifolate required for substrate activity for mammalian folyl-polyglutamate synthetase. Mol Pharmacol 27:156
Omura GA, Winton EF, Vogler WR, Zuckerman KS, Grillo-Lopez AJ (1983) Phase II study of amsacrine gluconate in refractory leukemia. Cancer Treat Rep 67:1131
Sasaki K, Hosoya R, Wang Y-M, Raulston GL (1983) Formation and disposition of 7-hydroxymethotrexate in rabbits. Biochem Pharmacol 32:503
Seglen PO (1973) Preparation of rat liver cells: III. Enzymatic requirements for tissue dispersion. Exp Cell Res 82:391
Seither RL, Rape TJ, Goldman ID (1989) Further studies on the pharmacologic effects of the 7-hydroxy catabolite of methotrexate in the L1210 murine leukemia cell. Biochem Pharmacol 38:815
Sholar PW, Baram J, Seither R, Allegra CJ (1988) Inhibition of folate-dependent enzymes by 7-OH-methotrexate. Biochem Pharmacol 37:3531
Slørdal L, Kolmannskog S, Prytz PS, Moe PJ, Aarbakke J (1986) Pharmacokinetics of methotrexate and 7-hydroxymethotrexate after high-dose (33.6 g/m2) methotrexate therapy. Pediatr Hematol Oncol 3:127
Spiegel RJ, Pizzo PA, Fantone JC, Zimmermann HJ (1980) Fatal hepatic necrosis after high-dose chemotherapy following haloalkane anesthesia. Cancer Treat Rep 64:1023
Steinberg SE, Campbell CL, Bleyer WA, Hillmann RS (1982) Enterohepatic circulation of methotrexate in rats in vivo. Cancer Res 42:1279
Wang Y-M, Fujimoto T (1984) Clinical pharmacokinetics of methotrexate in children. Clin Pharmacokinet 9:335
Yu D, Brasch H, Iven H (1989) No influence of enzyme inhibitors on the hydroxylation of methotrexate in rats. Cancer Lett 48:153
Author information
Authors and Affiliations
Additional information
This study was financially supported by the Norwegian Cancer Society and the Erna and Olav Aakre Foundation for Cancer Research
Rights and permissions
About this article
Cite this article
Bremnes, R.M., Smeland, E., Willassen, N.P. et al. Inhibition of 7-hydroxymethotrexate formation by amsacrine. Cancer Chemother. Pharmacol. 28, 377–383 (1991). https://doi.org/10.1007/BF00685693
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685693