Summary
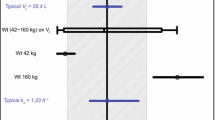
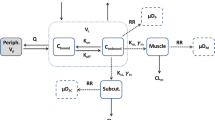
Suramin was given as an intravenous infusion to 16 cancer patients in a phase I trial. Individual pharmacokinetic parameters were calculated from a test dose given 1 week prior to the administration of a full-dose (350–700 mg/m2) regimen of 1-h loading and maintenance infusions. A distribution phase of 3.8 h was found. Plasma suramin concentrations were noted to increase following cessation of the intravenous test infusion in eight subjects. A model is proposed in which high-capacity, low-affinity binding of suramin to a shallow compartment adjacent to the intravascular space occurs rapidly during infusion, followed by absorption back into the measured blood pool with binding to plasma albumin. Despite the observable presence of this postinfusion peak shortly after the cessation of the brief suramin infusion, the pharmacokinetics of suramin were best characterized by a traditional two-compartment model. The dose-adjusted area under the concentration-time curve (AUC) increased with dose, supporting a hypothesis of sustained absorption of suramin to vascular endothelium but also raising the possibility of dose-dependent clearance.
Similar content being viewed by others
References
Akaike H (1976) An information criterion (AIC). Math Sci 14: 5–9
Bratt G, Tornebohm E, Lockner D, Bergstrom K (1985) A human pharmacological study comparing conventional heparin and low molecular weight heparin fragment. Thromb Haemost 53: 208–211
Cheson BD, Levine AM, Mildvan D, Kaplan LD, Wolfe P, Rios A, Groopman JE, Gill P, Volberding PA, Poiesz BJ, Gottlieb MS, Holden H, Volsky DJ, Silver SS, Hawkins MJ (1987) Suramin therapy in AIDS and related disorders: report of the U. S. Suramin Working Group. JAMA 258: 1347–1351
Collins JM, Klecker RW, Yarchoan R, Lane HC, Fauci AS, Redfield RR, Broder S, Myers CE (1986) Clinical pharmacokinetics of suramin in patients with HTLV-III/LAV infection. J Clin Pharmacol 26: 22–26
Cooper MR, Lieberman R, LaRocca RV, Gernt PR, Weinberger MS, Headlee DJ, Kohler DR, Goldspiel BR, Peck CC, Myers CE (1992). Adaptive control with feedback strategies for suramin dosing. Clin Pharmacol Ther 52: 11–23
deSwart CAM, Mijmeyer B, Roelofs JMM, Sixma JJ (1982) Kinetics of intravenously administered heparin in normal humans. Blood 60: 1251–1258
D'Argenio DZ, Schumitsky A (1990) ADAPT II user's guide. Biomedical Simulations Resource, University of Southern California Los Angeles
Egorin MJ, Van Echo DA, Whitacre MY, Forrest A, Sigman LM, Engisch KL, Aisner J (1986) Human pharmacokinetics, excretion and metabolism of the anthracycline antibiotic (7-OMEN, NSC 269 148) and their correlation with clinical toxicities. Cancer Res 46: 1513–1520
Harenberg J, Wurzner B, Zimmermann R, Schletter G (1986) Bioavailability and antagonization of the low molecular weight heparin CY 216 in man. Thromb Res 44: 549–554
Horne M, Stein CA, LaRocca RV, Myers C (1988) Circulating glycosaminoglycan anticoagulants associated with suramin treatment. Blood 71: 273–279
Hosang M (1985) Suramin binds to platelet-derived growth factor and inhibits its biological activity. J Cell Biochem 29: 265–273
Klecker RW, Collins JM (1985) Quantification of suramin by reverse-phase ion-pairing high-performance liquid chromatography. J Liquid Chromatogr 8: 1685–1696
LaRocca RV, Meer J, Gilliatt RW, Stein CA, Cassidy J, Myers CE, Dalakas MC (1990) Suramin induced acute polyneuropathy. Neurology 40: 954–960
Levine A, Gill P, Cohen J, Hawkins JG, Formenti SC, Aguilar S, Meyer PR, Krailo M, Parker J, Rasheed S (1986) Suramin antiviral therapy in the acquired immunodeficiency syndrome. Ann Intern Med 105: 32–37
Rago R, Tutsch K, Pomplun M, Hutson P, Wilding G (1992) Human plasma and tissue levels of suramin. Proc Am Assoc Cancer Res 33: A2559
Scher HI, Jodrell DI, Iversen JM, Curley T, Tong W, Egorin MJ, Forrest A (1992) Use of adaptive control with feedback to individualize suramin dosing. Cancer Res 52: 64–70
Stein CA, LaRocca RV, Thomas R, McAtee N, Myers CE (1989). Suramin: an anticancer drug with a unique mechanism of action. J Clin Oncol 7: 499–508
Stolzer TJ, LaFollette G, Gambertoglio J, Awuka F, Lin ET (1987) Determination of suramin in plasma and urine by ion-paired reversephase high-performance liquid chromatography. J Liquid Chromatogr 10: 3451–3462
WHO Expert Committee on Trypanosomiasis (1962) Technical Report Series 247, first report. WHO, Geneva
WHO Expert Committee on Onchocerciasis (1966) Technical Report Series 335, second report. WHO, Geneva
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hutson, P.R., Tutsch, K., Springgs, D. et al. Evidence of an absorption phase after short intravenous suramin infusions. Cancer Chemother. Pharmacol. 31, 495–499 (1993). https://doi.org/10.1007/BF00685042
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685042