Summary
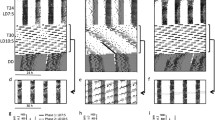
The circadian rhythm of wheel running behavior was observed to dissociate into two distinct components (i.e. ‘split’) within 30 to 110 days in 56% of male hamsters exposed to constant light (Figs. 1–2). Splitting was abolished in all 16 animals that were transferred from constant light (LL) to constant darkness (DD) within 1–4 days of DD, and the components of the re-fused activity rhythm assumed a phase relationship that is characteristic of hamsters maintained in DD (Figs. 3–5). Re-fusion of the split activity rhythm was accompanied by a change in period (τ); in 14 animals τ increased while in the other 2 animals τ decreased after transfer to DD.
After 10–30 days in DD, the hamsters were transferred back into LL at various time points throughout the circadian cycle. A few of these animals went through two or three LL to DD to LL transitions. The effect of re-exposure to LL was dependent on the phase relationship between the transition into LL and the activity rhythm. A rapid (i.e. 1–4 days) induction of splitting was observed in 7 of 9 cases when hamsters were transferred into LL 4–5 h after the onset of activity (Fig. 5). In the other 2 animals, the activity pattern was ultradian or aperiodic for 20 to 50 days before eventually coalescing into a split activity pattern. In contrast, transfer of animals (n = 13) from DD to LL at other circadian times did not result in the rapid induction of splitting and the activity rhythm continued to free-run with a single bout of activity (Fig. 5). Importantly, a transfer from DD to LL 4–5 h after the onset of activity did not induce splitting if the hamsters had not shown a split activity rhythm during a previous exposure to LL (n=10; Fig. 6).
These studies indicate that transfer of split hamsters from LL to DD results in the rapid re-establishment of the normal phase relationship between the two circadian oscillators which underlie the two components of activity during splitting. In addition, there appears to be a history-dependent effect of splitting which renders the circadian system susceptible to becoming split again. The rapid re-initiation of the split condition upon transfer from DD to LL at only a specific circadian time is discussed in terms of the phase response curve for this species.
Similar content being viewed by others
Abbreviations
- PRC :
-
phase response curve
References
Aschoff J (1954) Zeitgeber der tierischen Jahresperiodik. Naturwissenschaften 41:49–56
Aschoff J (1979) Orcadian rhythms: influences of internal and external factors on the period measured in constant conditions. Z Tierpsychol 49:225–249
Boulos Z, Terman M (1979) Splitting of circadian rhythms in the rat. J Comp Physiol 134:75–83
Daan S, Berde C (1978) Two coupled oscillators: simulations of the circadian pacemaker in mammalian activity rhythms. J Theor Biol 70:297–313
Daan S, Pittendrigh CS (1976) A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol 106:253–266
Elliott JA (1981) Circadian rhythms, entrainment and photoperiodism in the Syrian hamster. In: Follett BK, Follett DE (eds) Biological clocks in seasonal reproductive cycles. Wright, Bristol, pp 203–217
Ellis GB, McKlveen RE, Turek FW (in press) Dark pulses affect the circadian rhythm of activity in hamsters kept in constant light. Am J Physiol
Gwinner E (1974) Testosterone induces “splitting” of circadian locomotor activity rhythms in birds. Science 185:72–74
Hoffmann K (1971) Splitting of the circadian rhythm as a function of light intensity. In: Menaker M (ed) Biochronometry. Natl Acad Sci, Washington, DC, pp 134–150
Pittendrigh CS (1960) Circadian rhythms and the circadian organization of living systems. Cold Spring Harbor Symp Quant Biol 25:155–184
Pittendrigh CS (1967) Circadian rhythms, space research and manned space flight. In: Florkin M (ed) Life sciences and space research V. North-Holland, Amsterdam, pp 122–134
Pittendrigh CS (1974) Circadian oscillators in cells and the circadian organization of multicellular systems. In: The neurosciences: Third study program Schmitt FO, Worden FG (eds) MIT Press, Cambridge, MA, pp 437–458
Pittendrigh CS (1981) Circadian organization and the photoperiodic phenomena. In: Follett BK, Follett DE (eds) Biological clocks in seasonal reproductive cycles. Wright, Bristol, pp 1–35
Pittendrigh CS, Daan S (1976) A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker structure: A clock for all seasons. J Comp Physiol 106:291–331
Pittendrigh CS, Minis DH (1964) The entrainment of circadian oscillations by light and their role as photoperiodic clocks. Am Nat 98:261–294
Underwood H (1977) Circadian organization in lizards: The role of the pineal organ. Science 195:587–589
Author information
Authors and Affiliations
Additional information
This investigation was supported by NIH grants HD-09885 and HD-12622 from the National Institute of Child Health and Human Development and by a grant from the Whitehall Foundation
Recipient of Research Career Development Award K04 HD-00249 from the National Institute of Child Health and Human Development
Rights and permissions
About this article
Cite this article
Earnest, D.J., Turek, F.W. Splitting of the circadian rhythm of activity in hamsters: Effects of exposure to constant darkness and subsequent re-exposure to constant light. J. Comp. Physiol. 145, 405–411 (1982). https://doi.org/10.1007/BF00619345
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00619345