Abstract
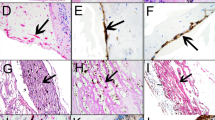
• Background: Epiretinal membranes from eyes with proliferative vitreoretinopathy (PVR) frequently express molecules associated with chronic inflammation. To investigate the extent to which inflammation may compromise the detached retina, we determined the expression of inflammatory molecules in anterior retina removed after relaxing retinotomy for retinal detachment complicated by anterior PVR. • Methods: Surgical retinal specimens were studied immunohistochemically for the distribution of the vascular cell adhesion molecules VCAM, E-selectin, P-selectin, ICAM and PECAM and for the presence of the cytokine TNFα and of T lymphocytes (CD3-positive cells), macrophages (CD68-positive cells) and HLA-DR molecules. The findings were compared with those in control cadaveric retina. • Results: Aberrant expresion of ICAM-1 was observed in four of nine retinal specimens from eyes with PVR, whereas its expression in control retinas was confined to the external limiting membrane and ganglion cell layers. PECAM was observed in seven of nine surgical retinal specimens and in four of five controls. E-selectin and P-selectin were expressed within the luminal aspects of four of nine retinal specimens from eyes with PVR, and VCAM was present in three of nine surgical specimens investigated. All cadaveric control retinas were negative for E-selectin and VCAM, whilst one was positive for P-selectin. Staining for TNFα was observed within luminal aspects and walls of retinal vessels from eight of nine surgical specimens, but was not seen in any of the cadaveric controls. T lymphocytes and cells expressing the macrophage marker CD68 were identified in two and seven of nine diseased retinas respectively, but not in any of the controls. Cells staining for HLA-DR were observed in eight of nine surgical retinal specimens and in three of five controls. • Conclusion: The present findings indicate that retina from eyes with advanced PVR may itself be subject to inflammatory changes, and indicate that the PVR process is not limited to retinal membranes, but involves a more widespread distribution of inflammation than is generally appreciated.
Similar content being viewed by others
References
Akira S, Hirano T, Taga T, Kishimoto T (1990) Biology of multifunctional cytokines: IL-6 and related molecules (IL-1 and TNF). FASEB J 4:2860–2867
Ayad S, Boot-Handford RP, Humphries MJ, Kadler KE, Shuttleworth CA (1994) The extracellular matrix Factsbook. Academic Press, London
Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA Jr (1987) Identification of an inducible endothelial-leukocyte adhesion molecule, ELAM-1. Proc Natl Acad Sci USA 84:9238–9242
Camussi G, Albano E, Tetta C, Bussolino F (1991) The molecular action of tumour necrosis factor-α. Eur J Biochem 202:3–14
Chandler DB, Hida T, Rozakis G, Forbes VS, Machemer R (1992) The lack of an effect of intraocular steroids on irradiated fibroblasts in experimental proliferative vitreoretinopathy. Graefe's Arch Clin Exp Ophthalmol 230:188–191
Charteris DG, Hiscott P, Grierson I, Lightman SL (1991) Proliferative vitreoretinopathy. Lymphocytes in epiretinal membranes. Ophthalmology 99:1364–1367
Cronstein BN, Weissmann G (1993) The adhesion molecules of inflammation. Arthritis Rheum 36:147–157
Esser P, Heimann K, Wiedemann P (1993) Macrophages in proliferative vitreoretinopathy and proliferative diabetic retinopathy: differentiation of subpopulations. Br J Ophthalmol 77:731–733
Federman JL, Eagle RC Jr (1990) Extensive peripheral retinectomy combined with posterior 360°C retinotomy for retinal reattachment in advanced proliferative vitreoretinopathy cases. Ophthalmology 97:1305–1320
Gilbert C, Hiscott P, Unger W, Grierson I, McLeod D (1988) Inflammation and the formation of epiretinal membranes. Eye [Suppl] 2:S140–156
Heidenkummer HP, Kampik A (1992) Intercellular adhesion molecule-1 (ICAM-1) and leukocyte function-associated antigen-1 (LEA-1) expression in human epiretinal membranes. Graefe's Arch Clin Exp Ophthalmol 230:483–487
Koch AE, Burrows JC, Haines GK, Carlos TM, Harlan JM, Leibovich SJ (1991) Immunolocalization of endothelial and leukocyte adhesion molecules in human rheumatoid and osteoarthritic synovial tissues. Lab Invest 64:313–320
Kohase MD, Henriksen-DeStefano D, May LT, Vilcek J, Sehgal PB (1986) Induction of beta, interferon by tumour necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell 45:659–666
Kovacs EJ (1991) Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today 12:17–23
Limb GA, Meager A, Woolley J, Wadhwa M, Biggerstaff J, Brown KA, Wolstencroft RA (1989) Release of cytokines during generation of lymphokine-activated killer (LAK) cells by IL-2. Immunology 68:514–519
Limb GA, Little BC, Meager A, Ogilvie JA, Wolstencroft RA, Franks WA, Chignell AH, Dumonde DC (1991) Cytokines in proliferative vitreoretinopathy. Eye 5:686–693
Limb GA, Franks WA, Munasinghe KR, Chignell AH, Dumonde DC (1993) Proliferative vitreoretinopathy: an examination of the involvement of lymphocytes, adhesion molecules and HLA-DR antigens. Graefe's Arch Clin Exp Ophthalmol 231:331–336
Limb GA, Alam A, Earley O, Green W, Chignell AH, Dumonde DC (1994) Distribution of cytokine proteins within epiretinal membranes in proliferative vitreoretinopathy. Curr Eye Res 13:791–798
Limb GA, Earley O, Jones SE, LeRoy F, Chignell AH and Dumonde DC (1994) Expression of mRNA coding for TNFα, IL-1 (3 and IL-6 by cells infiltrating retinal membranes. Graefe's Arch Clin Exp Ophthalmol 232:646–651
Machemer R, Aaberg TM, Freeman HM, Irvine AR, Lean JS, Michels RM (1991) An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol 112:159–165
Mackay CR, Imhof BA (1993) Cell adhesion in the immune system. Immunol Today 14:99–102
Mantovani A, Dejana E (1989) Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today 10:370–375
Matsushima K, Oppenheim JJ (1989) Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL1 and TNF. Cytokine 1:2–13
Montefort S, Holgate ST (1991) Adhesion molecules and their role in inflammation. Respir Med 85:91–99
Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R (1989) Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 59:1203–1211
Panayi GS (1992) The immunopathogenesis of rheumatoid arthritis. Rheumatol Rev 1:63–74
Pessara U, Koch N (1990) Tumor necrosis factor a regulates expression of the major histocompatibility complex class H- associated invariant chain by binding of an NF-KB-like factor to a promoter element. Mol Cell Biol 10:4146–4154
Pober IS, Gimbrone MA Jr, Lapierre LA, Mendrick DL, Fiers W, Rothlein R, Springer TA (1986) Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol 137:1893–1896
Postlethwaite AE, Seyer JM (1990) Stimulation of fibroblast chemotaxis by human recombinant tumour necrosis factor a (TNF-α) and a synthetic TNF-α 31-68 peptide. J Exp Med 172:1749–1756
Ryan SJ (1993) Traction retinal detachment. XLIX Edward Jackson memorial lecture. Am J Ophthalmol 115:1–20
Sethna F, Limb GA, Ellis B, Saundry R, Dumonde DC (1994) Cytokine binding to extracellular matrix proteins. Immunology 83 [Suppl]: 81
Stolpen AH, Guinan EC, Fiers W, Pober JS (1986) Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol 123:16–24
Wiedemann P, Weller M (1988) The pathophysiology of proliferative vitreoretinopathy. Acta Ophthalmol [Suppl] 189:7–15
Wilkinson LS, Edwards JCW (1991) Binding of antibodies raised against tumour necrosis factor alpha (TNFα) to blood vessels and macrophages in inflamed synovial tissue. Rheumatol Int 11:19–25
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Limb, G.A., Chignell, A.H., Woon, H. et al. Evidence of chronic inflammation in retina excised after relaxing retinotomy for anterior proliferative vitreoretinopathy. Graefe's Arch Clin Exp Ophthalmol 234, 213–220 (1996). https://doi.org/10.1007/BF00430412
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00430412