Abstract
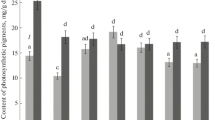
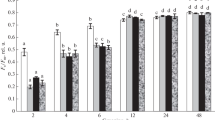
Changes in the levels of both ATP and protein in relation to Hill reaction activity following cold and dark storage and illumination of leaves of Lycopersicon esculentum Mill. were studied. Loss of Hill reaction activity observed during cold and dark storage of leaves for 3–4 days was accompanied by about 50% decrease of both ATP and protein levels while the content of chlorophyll was not affected Illumination of cold and dark stored leaves (8000 lx for 2 h) resulted in almost a complete restoration of both ATP and protein levels as well as Hill reaction activity. The latter process proceeded, however with different kinetics than the former ones. The rate of Hill reaction activity increase very rapidly from the beginning of illumination while the ATP level diminished during the first hour of illumination. In addition there was a lag in the increases in protein content. By about two hours of illumination all these processes reached the maximum values. Following illumination of leaf dises stored in the cold and dark in the presence of either cycloheximide or DCMU, both ATP and proteins levels as well as Hill reaction activity were greatly diminished. These data seem to suggest that the lack of reactivation of Hill reaction activity in the presence of these two inhibitors is due to inhibition of ATP synthesis required primarily for manganese reincorporation into the thylakoid membrane and theraby restoration of Hill reaction activity (Kaniuga, Zabek and Sochanowicz, Planta 1978b). Contribution of cytoplasmic protein synthesis in this process appears to be of secondary importance, although the inactivation and reactivation of electron transport are accompanied by a large loss (as high as 50%) and the restoration of the initial protein content in leaves following illumination.
Similar content being viewed by others
Abbreviations
- DCIP:
-
2,6-dichlorophenolindophenol
- DCMU:
-
3-(3,4-dichlorophenyl)-1,1 dimethylurea
- FFA:
-
free fatty acid
References
Cashmore, A.R.: Protein synthesis in plant leaf tissue. The sites of synthesia of the major proteins. J. Biol. Chem. 251, 2848–2853 (1976)
Choe, H.T., Thimann, K.V.: The restoration of photosynthetic activity by senescing chloroplasts of oat leaves. Planta 135, 101–107 (1977)
Frąckowiak-Sochanowicz, B., Nalęcz, M.J., Kaniuga, Z.: Zależność miedzy poziomem bialka i ATP a aktywnością reakcji Hilla w czasie przechowywania i reaktywacji liści roślin wrażliwych na przemarzanie. In: Abstr. 14th Meet. Polish Biochem. Soc. pp. 110–111, Lublin 1976
Goldthwaite, L., Laetch, W.M.: Regulation of senescence in been leaf discs by light and chemical growth regulators. Plant Physiol. 42, 1757–1762 (1967)
Guinn, G.: Changes in sugars, starch, RNA, protein and lipidsoluble phosphates in leaves of cotton plants at low temperature. Crop Sci. 11, 262–264 (1971)
Jones, P.C.T.: The effect of light, temperature and anaesthetics on ATP levels in the leaves of Chenopodium rubrum and Phaseolus vulgaris. J. Exp. Bot. 21, 58–63 (1970)
Kaniuga, Z., Michalski, W.: Photosynthetic apparatus in chilling-sensitive plants. II. Changes in free fatty acid composition and photoperoxidation in chloroplasts following cold storage and illumination of leaves in relation to Hill reaction activity. Planta 140, 129–136 (1978)
Kaniuga, Z., Sochanowicz, B., Ząbek, J., Krzystyniak, K.: Photosynthetic apparatus in chilling-sensitive plants. I. Reactivation of Hill reaction activity inhibited on the cold and dark storage of detached leaves and intact plants. Planta 140, 121–128 (1978a)
Kaniuga, Z., Ząbek, J., Sochanowicz, B.: Photosynthetic apparatus in chilling-sensitive plants. III. Contribution of loosely-bound manganese to the mechanism of reversible inactivation of Hill reaction activity following cold storage and illumination of leaves. Planta 144, 49–56 (1978b)
Kornberg, A., Horecker, B.L.: Glucose-6-phosphate dehydrogenase. In: Methods in enzymology, Vol. I. Preparation and assay of enzymes. pp. 323–327. Colowick, S.P., Kaplan, N.O., eds. New York: Academic Press 1955
Lowry, O.H., Rosenbrough, N.J., Farr, A.L., Randal, R.T.: Protein measurement with Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951)
Machold, O., Aurich, O.: Sites of synthesis of chloroplast lamellar proteins in Vicia faba. Biochim. Biophys. Acta 281, 103–112 (1972)
Martin, C., Thimann, K.V.: The role of protein synthesis in the senescence of leaves. I. The formation of protease. Plant Physiol. 49, 64–71 (1972)
McDonald, M.R.: Yeast hexokinase. In: Methods in enzymology. Vol. I. Preparation and assay of enzymes. pp. 269–276. Colowick, S.P., Kaplan, N.O., eds. New York: Academic Press 1955
McMahon, D.: Cycloheximide is not a specific inhibitor of protein synthesis in vivo. Plant Physiol. 55, 815–821 (1975)
Michalski, W., Tyll, J., Kaniuga, Z.: Rola galaktolipidów i wplyw egzogennej galaktolipazy na II układ fotosyntezy roślin wrażliwych na przemarzanie. In: Abstr. 14th Meet. Polish Biochem. Soc. pp. 44–45. Lublin 1976
Peterson, L.W., Huffacker, R.C.: Loss of ribulose 1,5-diphosphate carboxylase and increase in proteolytic activity during senescence of detached primary barley leaves. Plant Physiol. 55, 1009–1015 (1975)
Stewart, J.McD., Guinn, G.: Chilling injury and changes in adenosine triphosphate of cotton seedlings. Plant Physiol. 44, 605–608 (1969)
Stewart, J.McD. Guinn, G.: Chilling injury and nucleotides changes in young cotton plants. Plant Physiol. 48, 166–170 (1971)
Takegami, T.: A study on senescence in tabacco leaf discs. I. Inhibition by benzylaminopurine of decrease in protein level. Plant Cell Physiol. 16, 407–416 (1975)
Thimann, K.V., Tetley, R.M., Krivak, B.M.: Metabolism of oat leaves during senescence. V. Senescence in light. Plant Physiol. 59, 448–454 (1977)
Thomas, H., Stoddart, J.L.: Separation of chlorophyll degradation from other senescence processes in leaves of a mutant genotype of meadow fescue (Festuca pratensis L.). Plant Physiol. 56, 438–441 (1975)
Williamson, J.R., Corkey, B.E.: Assays of intermediates of the citric acid cycle and related compounds by fluorimetric methods. In: Methods in Enzymology. Vol. XIII. Citric acid cycle. pp. 434–515. Lowenstein, J.M., ed. New York: Academic Press 1969
Wilson, J.M.: Leaf respiration and ATP levels at chilling temperatures. New Phytol. 80, 325–334 (1978)
Wittenbach, V.A.: Induced senescence of intact wheat seedlings and its reversibility. Plant Physiol. 59, 1039–1042 (1977)
Wright, H., Simon, E.W.: Chilling injury in cucumber leaves. J. Exp. Bot. 24, 400–411 (1973)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sochanowicz, B., Kaniuga, Z. Photosynthetic apparatus in chilling-sensitive plants. Planta 144, 153–159 (1979). https://doi.org/10.1007/BF00387264
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00387264