Abstract
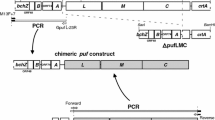
The genes encoding the α-and β-polypeptide subunits of the B806-866 membrane-bound light-harvesting complex of Chloroflexus aurantiacus have been cloned and the nucleotide sequences determined. The gene puf2A, which encodes the B806-866 α-polypeptide, began 28 bases downstream of the stop codon of puf2B, which encodes the B806-866 β gene. The gene-encoding cytochrome c-554, puf2C, was found about 250 bp downstream of puf2A. puf2A encoded a 13 amino acid extension at the C-terminus of the B806-866 α-polypeptide that was not present in the mature protein. These genes, unlike those of purple nonsulfur bacteria, did not form a contiguous operon with puf1L or puf1M, the genes encoding the L and M subunits of the photochemical reaction center. The occurrence of the two latter genes and of puf2B and puf2A in two separate operons has not been observed in purple bacteria. Under photoheterotrophic growth conditions, puf2B and puf2A were encoded on an abundant mRNA that was 0.5 kb long. Two monocistronic transcripts for puf2C were observed that had different 5′-ends. One transcript encoding all three genes was also detected. Nucleotide sequences very similar to the consensus promoter sequence of the Escherichia coli RNA polymerase σ70 subunit were found seven and eight bases upstream of the 5′-end of mRNA encoding puf2B and for one of the monocistronic mRNA encoding puf2C, respectively.
Similar content being viewed by others
Abbreviations
- Bchl :
-
Bacteriochlorophyll
References
Armstrong GA, Alberti M, Leach F, Hearst JE (1989) Nucleotide sequence, organization, and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus. Mol Gen Genet 216:254–268
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JT, Smith AJ, Strahl K (1988) Current protocols in molecular biology. Wiley, New York
Bauer C, Buggy J, Mosley C (1993) Control of photosystem genes in Rhodobacter capsulatus. Trends Genet 9:56–60
Bélanger G, Gingras G (1988) Structure and expression of the puf operon messenger RNA in Rhodospirillum rubrum. J Biol Chem 263:7639–7645
Belasco JG, Chen C-YA (1988) Mechanism of puf mRNA degradation: the role of an intercistronic stem-loop structure. Gene 72:109–117
Bérard J, Bélanger G, Corriveau P, Gingras G (1986) Molecular cloning and sequence of the of the B880 holochrome gene from Rhodospirillum rubrum. J Biol Chem 261:82–87
Castenholz RW, Pierson BK (1981) Isolation of members of the family Chloroflexaceae. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes. A handbook of habitats, isolation and identification of bacteria. Springer, Berlin Heidelberg New York, pp 290–298
Cech TR, Tanner NK, Tinoco I, Weir BR, Zuker M, Perlman PS (1983) Secondary structure of the Tetrahymena ribosomal RNA intervening sequence: structural homology with fungal mitochondrial intervening sequences. Proc Natl Acad Sci USA 80:3903–3907
Chung S, Frank G, Zuber H, Bryant DA (1994) Genes encoding two chlorosome components from the green sulfur bacteria Chlorobium vibrioforme 8327D and Chlorobium tepidum. Photosyn Res 41:261–275
Cozens AL, Walker JE (1987) The organization and sequence of the genes for ATP synthase subunits in the cyanobacterium Synechococcus 6301. Support for an endosymbiotic origin of chloroplasts. J Mol Biol 194:359–383
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12:387–395
Dracheva S, Williams JC, Van Driessche G, Van Beeumen JJ, Blankenship RE (1991) The primary structure of cytochrome c-554 from the green photosynthetic bacterium Chloroflexus aurantiacus. Biochemistry 30:11451–11458
Feick RG, Fitzpatrick M, Fuller RC (1982) Isolation and characterization of cytoplasmic membranes and chlorosomes from the green bacterium Chloroflexus aurantiacus. J Bacteriol 150: 905–915
Foster JM, Redlinger TE, Blankenship RE, Fuller RC (1986) Oxygen regulation of development of the photosynthetic membrane system in Chloroflexus aurantiacus. J Bacteriol 167:655–659
Gibson J, Ludwig W, Stackebrant E, Woese CR (1985) The phylogeny of the green photosynthetic bacteria: absence of a close relationship between Chlorobium and Chloroflexus. System Appl Microbiol 6:152–156
Højrup P, Gerola P, Hansen HF, Mikkelsen JM, Shahed AE, Knudsen J, Roepstorff P, Olson JM (1991) The amino acid sequence of a major protein component in the light harvesting complex of the green photosynthetic bacterium Chlorobium limicola f. thiosulfatophilum. Biochim Biophys Acta 1077: 220–224
Kawasaki ES (1990) Amplification of RNA. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 21–27
Kiley PJ, Kaplan S (1988) Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev 52:50–69
Klug G (1993) The role of mRNA degradation in the regulated expression of bacterial photosynthesis genes. Molec Microbiol 9:1–7
Ma D, Cook DN, O'Brien DA, Hearst JE (1993) Analysis of the promoter and regulatory sequences of an oxygen-regulated bch operon in Rhodobacter capsulatus by site-directed mutagenesis. J Bacteriol 175:2037–2045
Miller K (1987) Gel electrophoresis of RNA. BRL Focus 9:14–15
Nagashima KVP, Shimada K, Matsuura K (1993) Phylogenetic analysis of photosynthetic genes of Rhodcyclus gelatinosus: possibility of horizontal gene transfer in purple bacteria. Photosyn Res 36:185–191
Niedermeier G, Shiozawa JA, Lottspeich F, Feick R (1994) The primary structure of two chlorosome proteins from Chloroflexus aurantiacus. FEBS Lett 342:61–65
Olsen GJ, Woese CR, Overbeck R (1994) The winds of (evolutionary) change: breathing new life into microbiology. J Bacteriol 176:1–6
Ovchinnikov Yu A, Abdulaev NG, Zolotarev AS, Shmukler BE, Zargarov AA, Kutuzov MA, Telezhinskaya IN, Levina NB (1988a) Photosynthetic reaction centre of Chloroflexus aurantiacus. 1. Primary structure of L-subunit. JEBS Lett 231: 237–242
Ovchinnikov Yu A, Abdulaev NG, Shmuckler BE, Zargarov AA, Kutuzov MA, Telezhinskaya IN, Levina NB, Zolotarev AS (1988b) Photosynthetic reaction centre of Chloroflexus aurantiacus. Primary structure of M-subunit. FEBS Lett 232: 364–368
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Shine J, Dalgarno L (1974) The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci USA 71:1342–1346
Shiozawa JA, Lottspeich F, Oesterhelt D, Feick R (1989) The primary structure of the Chloroflexus aurantiacus reaction center polypeptides. Eur J Biochem 180:75–84
Shiozawa JA, Csiszàr K, Feick R (1990) Preliminary studies on the operon coding for the reaction center polypeptides in Chloroflexus aurantiacus. In: Drews G, Dawes EA (eds) Molecular biology of membrane-bound complexes in phototrophic bacteria, Plenum Press, New York, pp 11–18
Sprague SG, Staehelin LA, Fuller RC (1981) Semiaerobic induction of bacteriochlorophyll synthesis in the green bacterium Chloroflexus aurantiacus. J Bacteriol 147:1032–1039
Theroux SJ, Redlinger TE, Fuller RC, Robinson SJ (1990) Gene encoding the 5.7 kilodalton chlorosome protein of Chloroflexus aurantiacus: regulated message levels and a predicted carboxy terminal protein extension. J Bacteriol 172:4497–4504
Travers AA (1987) Structure and function of E. coli promoter DNA. CRC Crit Rev Biochem 22:181–219
Tullis RH, Rubin H (1980) Calcium protects DNase I from proteinase K: A new method for the removal of contaminating RNase from DNase I. Anal Biochem 107:260–264
Wagner-Huber R, Fischer U, Brunisholz R, Rümbeli M, Frank G, Zuber H (1990) The primary structure of the presumable BChl d-binding polypeptide of Chlorobium vibrioforme f. thiosulfatophilum. Z Naturforsch 45c:818–822
Wechsler T, Suter F, Fuller RC, Zuber H (1985a) The complete amino acid sequence of the bacteriochlorophyll c binding polypeptide of the green photosynthetic bacterium Chloroflexus aurantiacus. FEBS Lett 181:173–178
Wechsler T, Brunisholz R, Suter F, Fuller RC, Zuber H (1985b) The complete amino acid sequence of a bacteriochlorophyll a-binding polypeptide isolated from the cytoplasmic membrane of the green photosynthetic bacterium Chloroflexus aurantiacus. FEBS Lett 191:34–38
Wechsler TD, Brunisholz RA, Frank G, Suter F, Zuber H (1987) The complete amino acid sequence of the antenna polypeptide B806-865 β from the cytoplasmic membrane of the green bacterium Chloroflexus aurantiacus. FEBS Lett 210:189–194
Wellington CL, Bauer CE, Beatty JT (1992) Photosynthesis gene superoperons in purple nonsulfur bacteria: the tip of the iceberg? Can J Microbiol 38:20–27
Wiessner C, Dunger I, Michel H (1990) Structure and transcription of the genes encoding the B1015 light-harvesting complex β and α subunits and the photosynthetic reaction center L, M, and cytochrome c subunits from Rhodopseudomonas viridis. J Bacteriol 172:2877–2887
Woese CR (1987) Bacterial Evolution. Microbiol Rev 51:221–271
Zhu YS, Kaplan S (1985) Effects of light, oxygen and substrates on steady-state levels of mRNA coding for ribulose-1,5-bis phosphate carboxylase and light-harvesting and reaction center polypeptides in Rhodopseudomonas sphaeroides. J Bacteriol 162:926–932
Zuber H (1993) Structural features of photosynthetic light harvesting systems. In: Deisenhofer J, Norris JR (eds) The photosynthetic reaction center, vol 1. Academic Press, San Diego
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watanabe, Y., Feick, R.G. & Shiozawa, J.A. Cloning and sequencing of the genes encoding the light-harvesting B806-866 polypeptides and initial studies on the transcriptional organization of puf2B, puf2A and puf2C in Chloroflexus aurantiacus. Arch. Microbiol. 163, 124–130 (1995). https://doi.org/10.1007/BF00381786
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00381786