Summary
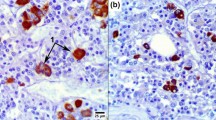
The morphological and functional changes associated with ageing were studied in adrenal steroidogenic cells derived from duck embryos. Cells grown for not more than three days had structural characteristics similar to their counterparts in vivo; they contained numerous lipid droplets and mitochondria, an abundant smooth edoplasmic reticulum, an even network of microtubules, and microfilaments that formed extensive and elaborate systems of parallel stress fibers. After the 3rd day of growth in culture, many of the cells started to decrease in size and become elongated; the older cells showed less well-defined actin filaments and contained elongated mitochondria, fewer lipid droplets, less smooth endoplasmic reticulum, and swollen cisternae of rough endoplasmic reticulum. The proliferative capacity of the cells was the same when they were cultured in either the presence or the absence of 1–24 ACTH. After the first day of growth in culture, the steroidogenic capacity of the cells declined and the addition of 1–24 ACTH to the growth medium did not prevent changes in their structure and function. The decline in steroidogenic capacity occurred both in terms of the amount of hormone released into the culture medium and in the ability of the cells to respond when incubated in buffer containing 1–24 ACTH. Since the basal unstimulated rates of corticosteroid production also declined as the cells aged, it is probable that the steroidogenic deficiency occurs at a site distal to the corticotropin receptor; this is also consistent with the ultrastructural observations that suggest a relationship between the morphological changes and the decline in steroidogenic capacity as the cells age.
Similar content being viewed by others
References
Arnold ML, James VHT (1971) Determination of deoxycorticosterone in plasma; double isotope and immunoassay methods. Steroids 18:789–891
Carsia RV, Scanes CG, Malamed S (1985) Loss of sensitivity to ACTH of adrenocortical cells isolated from maturing domestic fowl. Proc Soc Exp Biol Med 179:279–282
Carsia RV, Morin ME, Rosen HD, Weber H (1987) Ontogenic corticosteroidogenesis of the domestic fowl: response of isolated adrenocortical cells. Proc Soc Exp Biol Med 184:436–445
Cathiard AM, Crozat A, Durand P, Saez JM (1985) In vitro spontaneous and adrenocorticotropin-dependent maturation of the steroidogenic pathway of ovine fetal adrenal cells. Endocrinology 116:585–590
Cheitlin R, Ramachandran J (1981) Regulation of actin in rat adrenocortical renocortical cells by corticotropin. J Biol Chem 256:3156–3158
Collie MA, Cronshaw J, Holmes WN (1992)_A comparison of the responses of dispersed steroidogenic cells derived from embryonic adrenal tissue from the domestic chicken (Gallus domesticus), the domestic Pekin duck and the wild mallard duck (Anas platyrhynchos), and the domestic muscovy duck (Cairina moschata). Gen Comp Endocrinol (in press)
Crivello JF, Hornsby PJ, Gill GN (1982) Metyrapone and antioxidants are required to maintain aldosterone systhesis by cultured bovine adrenocortical zona glomerulosa cells. Endocrinology 111:469–479
Cronshaw J, Holmes WN, Ely JA, Redondo JL (1989) Pre-natal development of the adrenal steroidogenic tissue in the Mallard duck (Anas platyrhynchos). Cell Tissue Res 258:593–601
Cronshaw J, Reese BK, Collie MA, Holmes WN (1992) Cytoskeletal changes accompanying ACTH-induced steroidogenesis in cultured embryonic adrenal gland cells from the Pekin duck. Cell Tissue Res 268:1–9
Di Blasio A, Fujii D, Yamamoto M, Martin M, Jaffe R (1990) Maintenance of cell proliferation and steroidogenesis in cultured human fetal adrenal cells chronically exposed to adrenocorticotropic hormone: rationalization of in vitro and in vivo findings. Biol Reprod 42:683–691
Donaldson EM, Holmes WN, Stachenko J (1965) In vitro corticosteroidogenesis by the duck (Anas platyrhynchos) adrenal. Gen Comp Endocrinol 5:542–551
Dupperay A, Chambaz EM (1980) Effect of prostaglandin E1 and ACTH on proliferation and steroidogenic activity of bovine adrenocortical cells in primary culture. Steroid Biochem 13:1359–1364
Durand P, Cathiard AM, Saez JM (1984) In vitro maturation of steroidogenic capacity of ovine fetal and neonatal adrenal cells. Endocrinology 114:1128–1134
Gill GN, Ill CR, Simonian MH (1977) Angiotensin stimulation of bovine adrenocortical cell growth. Proc Natl Acad Sci USA 74:5569–5573
Goodyer CG, Torday JS, Smith BT, Giroud CJP (1976) Preliminary observations of bovine adrenal fasciculata-reticularis cells in monolayer culture: steroidogenesis, effects of ACTH and cyclic AMP. Acta Endocrinol 83:373–385
Gospodarowicz D, Ill CR, Hornsby PJ, Gill GN (1977) Control of bovine adrenal cortical cell proliferation by fibroblast growth factor. Lack of effect of epidermal growth factor. Endocrinology 100:1080–1089
Heikkila P, Kahri AI, Ehnholm C, Kovanen PT (1989) The effect of low- and high-density lipoprotein cholesterol on steroid hormone production and ACTH-induced differentiation of rat adrenocortical cells in primary culture. Cell Tissue Res 256:478–494
Heikkila P, Kahri AI, Ehnholm C, Kovanen PT (1990) Effects of mevinolin, an inhibitor of cholesterol synthesis, on the morphology and function of differentiating and differentiated rat adrenocortical cells in primary culture. Cell Tissue Res 261:125–132
Higuchi T, Kaneko A, Abel JH Jr, Niswender GD (1976) Relationship between membrane potential and progesterone release in ovine corpora lutea. Endocrinology 99:1023–1032
Holmes WN, Al-Ghawas SC, Cronshaw J, Rohde KE (1991) The structural organization and the steroidogenic responsiveness in vitro of adrenal gland tissue from the neonatal mallard duck (Anas platyrhynchos). Cell Tissue Res 263:557–566
Holmes WN, Cronshaw J, Collie MA, Rohde KE (1992) Cellular aspects of the stress response in precocial neonates. Ornis Scand (in press)
Hornsby PJ (1980) Regulation of cytochrome P-450-supported 11-beta-hydroxylation of deoxycortisol by steroids, oxygen, and antioxidants in adrenocortical cell cultures. J Biol Chem 255:4020–4027
Hornsby PJ (1985) Regulation of adrenocortical cell proliferation in culture. Endocrinol Res 10:259–281
Hornsby PJ, Gill GN (1977) Hormonal control of adrenocortical cell proliferation. J Clin Invest 60:342–352
Hornsby PJ, Gill GN (1978) Characteristics of adult bovine adrenocortical cells throughout their life span in tissue culture. Endocrinol 102:926–936
Hornsby PJ, Gill GN (1981) Regulation of responsiveness of cultured adrenal cells to adrenocorticotropin and prostaglandin E1: cell density, cell division, and inhibitors of protein synthesis. Endocrinology 108:183–188
Hornsby PJ, O'Hare MJ, Neville AM (1973) Effect of ACTH on biosynthesis of aldosterone and corticosterone on monolayer cultures of rat adrenal zona glomerulosa cells. Biochem Biophys Res Comm 54:1544–1559
Hornsby PJ, O'Hare MA, Neville AM (1974) Functional and morphological observation on rat adrenal zona glomerulosa cells in monolayer culture. Endocrinology 95:1240–1251
Hornsby PJ, Simonian MH, Gill GN (1980) Ageing of adrenocortical cells in culture. Int Rev Cytology 10:131–162
Hornsby PJ, Sturek H, Harris SE, Simmonian MH (1983) Serum and growth factor requirements for proliferation of human adrenocortical cells in culture: comparison with bovine adrenocortical cells. In Vitro 19:863–869
Hornsby PJ, Ryan RF, Cheng CY (1989) Replicative senescence and differentiated gene expression in cultured adrenocortical cells. Exp Gerontology 24:539–558
Kahri AI, Heikkila P, Ehnholm R, Kovanen PT (1989) Sequential expression of high and low density lipoprotein receptors in differentiating fetal rat adrenocortical cells in primary culture. Endocrinology 125:68–75
Kawamura M, Nakamichi N, Imagawa N, Tanaka Y, Tomita C, Matsuba M (1984) Effect of adrenaline on steroidogenesis in primary cultured bovine adrenocortical cells. Jpn J Pharmacol 36:35–41
Kramer R, McCarthy J, Simpson E, Waterman M (1983) Effects of ACTH on steroidogenesis in bovine adrenocortical cells in primary culture-increased secretion of 17-alpha-hydroxylated steroids associated with a refractoriness in total steroid output. J Steroid Biochem 18:715–723
Liles S, Ramachandran J (1977) Regulation of 5-ene-3-beta hydroxysteroid dehydrogenase-isomerase activity in adrenocortical cell cultures by adrenocorticotropin. Biochem Biophys Res Comm 79:226–233
Masui H, Garren LD (1971) Inhibition of replication in functional mouse adrenal tumor cells by adrenocorticotrophic hormone mediated by adenosine 3′5′-cyclic monophosphate. Proc Natl Acad Sci USA 68:3206–3211
Mattson P, Kowal J (1980) Acute steroidogenic stimulation of cultured adrenocortical tumor cells: an electron microscopic analysis. Tissue Cell 12:685–701
Mattson P, Kowal J (1982) Effects of cytochalasin B on unstimulated and adrenocorticotropin-stimulated adrenocortical tumor cells in vitro. Endocrinology 111:1632–1647
Mayes DM, Furuyama S, Kem DC, Nugent CA (1970) Radioimmunoassay of aldosterone. J Clin Endocrinol 30:682–685
Morera AM, Saez JM (1980) Mechanisms implicated in ACTH-induced steroidogenesis and DNA synthesis refractoriness on adrenal mouse cell line (Y1). J Steroid Biochem Exp Cell Res 127:446–451
Neville AM, O'Hare MJ (1978) Cell culture and histopathology of the human adrenal cortex in relation to hypercorticalism. In: James VHT, Serio M, Giusti G, Martini L (eds) The endocrine function of the human adrenal cortex. Academic Press, London, pp 229–249
Nishida S, Matsamura S, Horino M, Oyama H, Tenku A (1976) A radioimmunoassay for human plasma corticosterone. Endocrinol Jpn 23:465–469
O'Hare MJ, Neville AM (1973a) Morphological responses to corticotrophin and cyclic AMP by adult rat adrenocortical cells in monolayer culture. J Endocrinol 56:529–536
O'Hare MJ, Neville AM (1973b) The steroidogenic response of adult rat adrenocortical cells in monolayer culture. J Endocrinol 56:537–549
Pudney J, Sweet PR, Vinson GP, Whitehouse BJ (1981) Morphological correlates of hormone secretion in the rat adrenal cortex and the role of filopodia. Anat Rec 201:537–551
Provencher P, Lorrain A, Belanger A, Fiet J (1990) Steroid biosynthesis by zona glomerulosa-fasciculata cells in primary culture of guinea-pig adrenals. J Steroid Biochem 36:589–596
Rainey W, Hornsby PJ, Shay J (1983) Morphological correlates of adrenocorticotropin-stimulated steroidogenesis in cultured adrenocortical cells: differences between bovine and human adrenal cells. Endocrinology 113:48–54
Ramachandran J, Suyama T (1975) Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotrophin. Proc Natl Acad Sci USA 72:113–118
Redondo JL, Cronshaw J, Holmes WN, Lin FL (1989) Structural and functional characteristics of primary cell cultures derived from the adrenal gland tissue of neonatal mallard ducklings (Anas platyrhynchos). Cell Tissue Res 257:389–397
Ryan RF, Hancock JP, McDonald JJ, Hornsby PJ (1989) Cellular senescence involves stochastic processes causing loss of expression of differentiated function genes: visualization by in situ hybridization for steroid 17-alpha-hydroxylase in bovine adrenocortical cells. Exp Cell Res 180:36–48
Rybak S, Ramachandran J (1982) Mechanism of induction of 5-ene-3-beta-hydroxysteroid dehydrogenase-isomerase activity in rat adrenocortical cells by corticotropin. Endocrinology 111:427–433
Simonian MH, Gill GN (1979) Regulation of deoxyribonucleic acid synthesis in bovine adrenocortical cells in culture. Endocrinology 104:588–593
Simonian MH, Gill GN (1981) Regulation of the fetal human adrenal cortex: Effects of adrenocorticotropin on growth and function of monolayer cultures of fetal and definitive zone cells. Endocrinology 108:1769–1779
Simonian MH, Hornsby PJ, Ill CR, O'Hare MJ, Gill GN (1979) Characterization of cultured bovine adrenocortical cells and derived clonal lines: regulation of steroidogenesis and culture life span. Endocrinology 105:99–108
Simonian MH, White ML, Gill GN (1982) Growth and function of cultured adrenocortical cells in a serum-free defined medium. Endocrinology 111:919–927
Slavinski EA, Auersperg N (1976) The role of extracellular matrix in controlling the phenotypic expression of normal adult rat adrenal cortical cells in vitro. J Cell Biol 70:314a
Slavinski EA, Auersperg N, Jull JW (1974) Propagation in vitro of functional rat adrenal cortical cells: modifications of the differentiated state by culture conditions. In Vitro 9:260–269
Slavinski-Turley EA, Auersperg N (1978) Cultured adrenocortical cells in various states of differentiation: electronmicroscopic characterization and ultrastructural response to adrenocorticotropin. J Endocrinol 78:427–434
Williams BC, Lightly ERT, Ross AR, Bird IM, Walker SW (1989) Characterization of the steroidogenic responsiveness and ultrastructure of purified zona fasciculata/reticularis cells from bovine adrenal cortex before and after primary culture. J Endocrinol 121:317–324
Yang LL, Hornsby PJ (1989) Dissociation of senescence-associated changes in differentiated gene expression and replicative senescence in cultured adrenocortical cells. J Cell Sci 94:757–768
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cronshaw, J., Collie, M.A. & Holmes, W.N. Functional and morphological changes associated with the ageing of primary cultures of embryonic adrenal gland cells derived from the Pekin duck. Cell Tissue Res 269, 535–545 (1992). https://doi.org/10.1007/BF00353908
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00353908