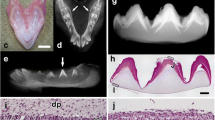
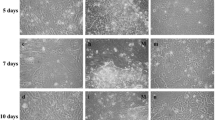
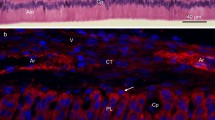
Summary
Enamel is the hardest tissue in vertebrates. Ameloblasts are derived from epithelial cells and are responsible for enamel formation. They secrecte enamel matrix components in which amelogenins are the major proteins, the biochemical properties of which are well known. However, little is known about the characteristics of ameloblasts themselves or about the functions of amelogenins. In this study, we developed a novel primary and secondary culture system for ameloblasts using a monoclonal antibody which recognized amelogenin (En3). The cell layer on dentine removed from rat mandibular incisors was isolated and cultured in low calcium, serum-free medium. Primary culture was performed on collagen-coated culture plates and typically, two types of cells appeared. One major type changed morphology after the addition of a high concentration of calcium to the medium. Expression of amelogenin was shown as cytoplasmic particles in these cells using En3. In the secondary culture, expression of amelogenins was also observed. In this system, the cells grew and maintained the expression of amelogenin for about 3 weeks.
Similar content being viewed by others
References
Zeicher-David M, Slavkin HC, Lyaruu DM, Termine JD (1983) Biosynthesis and secretion of enamel proteins during hamster tooth development. Calcif Tissue Int 35:366–371
Herold RC, Boyde A, Rosenbloom J, Lally ET (1987) Monoclonal antibody and immunogold cytochemical of amelogenins in bovine secretory amelogenesis. Arch Oral Biol 32:439–444
Termine JD, Belcourt AB, Christner PJ, Conn KM, Nylen MV (1980) Properties of dissociatively extracted fetal tooth matrix proteins. I. Principal molecular species in developing bovine enamel. J Biol Chem 255:9760–9768
Takagi T, Suzuki M, Baba T, Minegishi K, Sasaki S (1984) Complete amino acid sequence of amelogenin in developing bovine enamel. Biochem Biophys Res Commun 121:592–597
Snead ML, Lau EC, Zeichner-David M, fincham AG, Woo SLC, Slavkin HC (1985) DNA sequence for cloned cDNA for murine amelogenin reveals the amino acid sequence for enamel-specific protein. Biochem Biophys Res Commun 121:812–818
Shimokawa H, Sobel ME, Sasaki M, Termine JD, Young MF (1987) Heterogeneity of amelogenin mRNA in the bovine tooth germ. J Biol Chem 262:4042–4047
Shimokawa H, Sasaki S (1978) Study on the biosynthesis of bovine enamel protein in vitro. J Dent Res 57:133–138
Rheinwald JG, Green H (1975) Serial cultivation of strains of human epidermal keratinocytes. Cell 6:331–344
Peehi DM, Ham RG, (1980) Growth and differentiation of human keratino-cytes without a feeder layer on conditioned medium. In Vitro 16:516–525
Peehi DM, Ham RG (1980) Clonal growth of human keratinocytes with small amounts of dialyzed serum. In vitro 16:526–538
Hawley Nelson P, Sullivan JE, Kung M, Henning H, Yuspa SH (1980) Optimized conditions for the growth of human epidermal cells in culture. J Invest Dermatol 75:176–182
Henning H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa S (1980) Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell 19:245–254
Inai T, Kukita T, Ohsaki Y, Nagata K, Kukita A, Kurisu K (1991) Immunohistochemical demonstration of amelogenin penetration toward the dental pulp in the early stages of ameloblast development in rat molar tooth germs. Anat Rec 229:259–270
Matsuhashi S, Sugihara H (1984) Development of chicken epidermis cultured with embryo extract. Virchows Arch [Cell Pathol] 46:53–64
Snead M, Lau WEC, Slavkin HC (1988) Spatial-and temporalrestricted pattern for amelogenin gene expression during mouse molar tooth organogenesis. Development 104:77–85
Breitkreutz D, Bohnert A, Herzmann E, Bowden PE, Boukamo P, Fusenig NE (1984) Differentiation specific functions in cultured and tranplanted mouse keratinocytes: environmental influences on ultrastructure and keratin expression. Differentiation 26:154–169
Roop DR, Hawley-Nelson P, Cheng CK, Yuspa SH (1983) Keratin gene expression in mouse epidermis and cultured epidermal cells. Proc Natl Acad Sci USA 80:716–720
Roop DR, Huitfeldt H, Kikenny A, Yuspa SH (1987) Regulated expression of differentiation-associated keratins in cultured epidermal cells detected by monospecific antibodies to unique peptides of mouse epidermal keratins. Differentiation 35:143–150
Yuspa SH, Kikenny AE, Steinert PM Roop DR (1989) Expression of murine epidermal differentiation markers is tightly regulated extracellular calcium concentration in vitro. J Cell Biol 109:1207–1217
Takeichi M, Okada TS (1972) Roles of magnesium and calcium ions in cell-to-substrate adhesion. Exp Cell Res 74:51–60
Slavkin HC, Bessem C, Bringas P, Zeichner-David M, Nanci A, Snead ML (1988) Sequential expression and differential function of multiple enamel proteins during fetal, neonatal, and early postnatal stages of mouse molar organogenesis. Differentiation 37:26–39
Nanci A, Ahluwalia JP, Pompura JR, Smith CE (1989) Biosynthesis and secretion of enamel proteins in rat incisor. Anat Rec 224:272–291
Inage T, Shimokawa H, Teranishi Y, Iwase T, Toda Y, Moro I (1989) Immunocytochemical demonstration of amelogenins and enamelins secreted by ameloblasts during the secretory and maturation stages. Arch Histol Cytol 52:213–229
Smith CE, Nanci A (1989) Secretory activity as a function of the development and maturation of ameloblasts. Connect Tissue Res 22:147–156
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kukita, A., Harada, H., Kukita, T. et al. Primary and secondary culture of rat ameloblasts in serum-free medium. Calcif Tissue Int 51, 393–398 (1992). https://doi.org/10.1007/BF00316886
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00316886