Summary
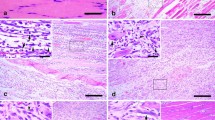
Degenerating and regenerating muscle fibers, in serotonin-induced myopathy (SM) of rats, were investigated histochemically, immunohistochemically and electron microscopically with polyclonal antibodies against dystrophin, type IV collagen and laminin. The myopathy produced was characterized by grouping of degenerating and regenerating muscle fibers, and degeneration of capillary endothelial cells. Dystrophin disappeared in an early stage of muscle degeneration and reappeared in an early stage of regeneration. On the other hand, type IV collagen and laminin were well preserved throughout the degeneration and regeneration processes, even on the shrunk and wrinkled basement membrane of empty muscle fibers after phagocytosis. Muscle fiber regeneration was completed within each tube of the preserved basement membrane through the fusion of myoblasts derived from satellite cells of single necrotic fibers, myotubes already being visible on the 1st or 2nd day of regeneration on light microscopy. These small regenerating myotubes did not fuse with each other at all. The findings in the present experimental SM study are compatible with those in Duchenne muscular dystrophy, especially at the preclinical stage.
Similar content being viewed by others
References
Appenzellar O, Ogin G (1975) Pathogenesis of muscular dystrophies. Arch Neurol 32: 2–4
Arahata K, Ishiura S, Ishiguro T, Tsukahara T, Suhara Y, Eguchi C, Ishihara T, Nonaka I, Ozawa E, Sugita H (1988) Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature 333: 861–863
Arora RC, Kuncl RW, Morgan J, Cohen L, Meltzer HY (1987) Serotonin uptake in blood platelets of Duchenne muscular dystrophy patients. Muscle Nerve 10: 359–362
Duncan CJ (1989) Dystrophin and the integrity of the sarcolemma in Duchenne muscular dystrophy. Experimentia 45: 175–177
Engel WK (1967) Muscle biopsies in neuromuscular diseases. Pediatr Clin North AM 14: 963–995
Hathaway PW, Engel WK, Zellweger H (1970) Experimental myopathy after microarterial embolization: comparison with childhood X-linked pseudohypertrophic muscular dystrophy. Arch Neurol 22: 365–378
Hoffman EP, Brown RH Jr, Kunkel LM (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51: 919–928
Ishiura S, Arahata K, Tsukahara T, Koga R, Anraku H, Yamaguchi M, Kikuchi T, Nonaka I (1990) Antibody against the C-terminal portion of dystrophin crossreacts with the 400-kDa protein in the pia mater of dystrophin-deficient mdx mouse brain. J Biochem (in press)
Jerusalem F, Engel AG, Gomez MR (1974) Duchenne dystrophy. I. Morphometric study of the muscle microvasculature. Brain 97: 115–122
Koehler J (1977) Blood vessel structure in Duchenne muscular dystrophy. I. Light and electron microscopic observations in resting muscle. Neurology 27: 861–868
Mendell JR, Engel WK, Derrer EG (1972) Increased plasma enzyme concentrations in rats with functional ischemia of muscle provide a possible model of Duchenne muscular dystrophy. Nature 239: 522–524
Miike T (1983) Maturational defect of regenerating muscle fibers in cases with Duchenne and congenital muscular dystrophies. Muscle Nerve 6: 545–552
Miike T, Sugino S, Ohtani Y, Taku K, Yoshioka K (1987) Vascular endothelial cell injury and platelet embolism in Duchenne muscular dystrophy at the preclinical stage. J Neurol Sci 82: 67–80
Miike T, Miyatake M, Zhao J, Yoshioka K, Uchino M (1989) Immunohistochemical dystrophin reaction in synaptic regions. Brain Dev 11: 344–346
Miyatake M, Miike T, Zhao J, Yoshioka K, Uchino M, Usuku G (1989) Possible systemic smooth muscle dysfunction due to a deficiency of dystrophin in Duchenne muscular dystrophy. J Neurol Sci 93: 11–17
Miyatake M, Miike T, Zhao J, Yoshioka K, Uchino M, Usuku G (1990) Dystrophin: localization and presumed function. Muscle Nerve (in press)
Munsat TL, Hudgson P, Johnson MA (1977) Experimental serotonin myopathy, Neurology 27: 772–782
Murphy DL, Mendell JR, Engel WK (1973) Serotonin and platelet function in Duchenne muscular dystrophy. Arch Neurol 28: 239–242
Nonaka I, Takagi A, Ishiura S, Nakase H, Sugita H (1983) Pathophysiology of muscle fiber necrosis induced by bupivacaine hydrochloride (Marcaine). Acta Neuropathol (Berl) 60: 167–174
Parker JM, Mendell JR (1974) Proximal myopathy induced by 5-HT-imipramine simulates Duchenne dystrophy. Nature 247: 103–104
Rowland LP (1979) Chemistry and chemical pathology of sarcolemma. In: Aguayo AJ, Karpati G (eds) Current topics in nerve and muscle research. Excerpta Medica, Amsterdam, pp 16–28
Smith GM (1989) Involvement of S-hydroxytryptamine in platelet aggregation in vivo in rats and guinea pigs. Thromb Haemost 61: 463–467
Tanabe Y, Esaki K, Nomura T (1986) Skeletal muscle pathology in X chromosome-linked muscular dystrophy (mdx) mouse. Acta Neuropathol (Berl) 69: 91–95
Vracko R, Bendtt EP (1972) Basal lamina: the scaffold for ordinary cell replacement. J Cell Biol 55: 406–419
Wright TL, O'Neill AJ, Olson WH (1973) Abnormal intrafibrillar monoamines in sex-linked muscular dystrophy. Neurology 23: 510–517
Zubrycka-Gaarn EE, Bulman DE, Karpati G, Burghes AMH, Belfall B, Klamut HJ, Talbot J, Hodges RS, Ray PN, Worton RG (1988) The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature 333: 466–469
Author information
Authors and Affiliations
Additional information
This work was supported in part by Grant no. 83-05 from the National Center for Nervous, Mental and Muscular Disorders (NCNMMD) of the Ministry of Health and Welfare, Japan
Rights and permissions
About this article
Cite this article
Narukami, H., Yoshioka, K., Zhao, J. et al. Experimental serotonin myopathy as an animal model of muscle degeneration and regeneration in muscular dystrophy. Acta Neuropathol 81, 510–516 (1991). https://doi.org/10.1007/BF00310131
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00310131