Abstract
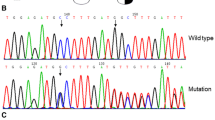
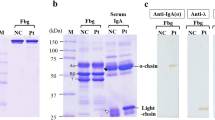
We discovered a congenital heterozygous dysfibrinogen in a patient and reported this case in relation to surgery some time ago (Jpn J Surg (1988) 18:43–46).3 Further studies on the isolated abnormal population of fibrinogen derived from this patient have revealed that fibrinopeptide A was not cleaved by ancrod, a snake venom-derived thrombin-like enzyme, but by thrombin, slowly but completely. The released fibrinopeptide A components, being the A, AY, and AP peptides, were all found to be abnormal, as evidenced by slightly earlier elution positions on high-performance liquid chromatography, compared with the normal counterparts. By analyzing their amino acid sequence, we have identified an arginine to histidine substitution at position 16 of the Aα chain, the thrombin cleavage site. Utilizing insolubilized abnormal fibrinogen, we confirmed that the polymerization site assigned to the central E domain, the “A” site, was exposed by thrombin, but not by ancrod. This dysfibrinogen, designated as fibrinogen Osaka IV, is the second abnormal molecule with an Aα arginine-16 to histidine substitution identified among Japanese families.
Similar content being viewed by others
References
Southan C (1988) Molecular and genetic abnormalities of fibrinogen. In: Francis JL (ed) Fibrinogen, Fibrin Stabilisation, and Fibrinolysis. Ellis Horwood Chichester, UK, pp 65–99
Matsuda M, Yoshida N, Terukina S, Yamazumi K, Maekawa H (1990) Molecular abnormalities of fibrinogen — The present status of structure elucidation. In: Matsuda M, Iwanaga A, Takada A, Henschen A (eds) Fibrinogen 4, Current basic and clinical aspects. Elsevier Amsterdam, pp 39–52
Tsujinaka T, Kanbayashi J, Kang J, Sakon M, Mori T (1988) Intra-operative management of a dysfibrinogenic patient with gastric cancer. Jpn J Surg 18:43–46
Kudryk B, Blombäck B, Blombäck M. (1976) Fibrinogen Detroit — an abnormal fibrinogen with non-functional NH2-terminal polymerization domain. Thromb Res 9:25–36
Matsuda M, Baba M, Morimoto M, Nakamikawa C. (1983) Fibrinogen Tokyo II: An abnormal fibrinogen with an impaired polymerization site on the aligned DD domain of fibrin molecules. J Clin Invest 72:1034–1041
Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Yamazumi K, Terukina S, Onohara S, Matsuda M. (1988) Normal plasmic cleavage of the γ-chain variant of fibrinogen Saga with an Arg-275 to His substitution. Thromb Haemost 60:476–480
Matsuda M, Saeki E, Kasamatsu A, Nakamikawa C, Manabe C, Samejima Y. (1985) Fibrinogen Kawaguchi: An abnormal fibrinogen characterized by defective release of fibrinopeptide A. Thromb Res 37:379–390
Kehl M, Lottspeich F, Henschen A. (1981) Analysis of human fibrinopeptides by high-performance liquid chromatography. Z Physiol Chem 362:1661–1664
Bildlingmeyer BA, Cohen SA, Tarvin TL. (1984) Rapid analysis of amino acids using pre-column derivatization. J Chromatogr 336:93–104
Yamazumi K, Shimura K, Terukina S, Takahashi N, Matsuda M. (1989) A γ methionine-310 to threonine substitution and consequent N-glycosilation at γ asparagine-308 identified in a congenital dysfibrinogenemia associated with post-traumatic bleeding, fibrinogen Asahi. J Clin Invest 83:1590–1597
Terukina S, Matsuda M, Hirata H, Takeda Y, Miyata T, Takao T, Shimonishi Y. (1988) Substitution of γArg-275 by Cys in an abnormal fibrinogen, fibrinogen Osaka II. Evidence for a unique solitary cystine structure at the mutation site. J Biol Chem 263:13679–13587
Rixon MW, Chan W-Y, Davie EW, Chung DW. (1983) Characterization of a complementary deoxyribonucleic acid coding for the α chain of human fibrinogen. Biochemistry 22:3237–3244
Asakura S, Terukina S, Yamazumi K, Matsuda M, Murayama H, Higuchi A, Musashi M, Miyazaki T. (1989) Fibrinogen Sapporo: Dysfibrinogenemia characterized by the replacement of Aα arginine-16 by histidine resulting in the delayed release of fibrinopeptide A by thrombin. Acta Haematol Jpn 52:1094–1104
Olexa SA, Budzynski AZ. (1980) Evidence for four different polymerization sites involved in human fibrin formation. Proc Natl Acad Sci USA 77:1374–1378
Baker D, Schafer M, White R. (1984) Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell 36:131–138
Miyata T, Terukina S, Matsuda M, Kasamatsu A, Takeda Y, Murakami T, Iwanaga S. (1987) Fibrinogens Kawaguchi and Osaka: An amino acid substitution of Aα arginine-16 to cysteine which forms an extra interchain disulfide bridge between the Aα chains. J Biochem 102:93–101
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamazumi, K., Terukina, S., Matsuda, M. et al. Fibrinogen osaka IV: A congenital dysfibrinogenemia found in a patient originally reported in relation to surgery, now defined to have an Aα arginine-16 to histidine substitution. Surg Today 23, 45–50 (1993). https://doi.org/10.1007/BF00308999
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00308999