Abstract
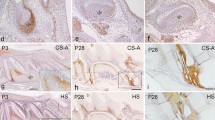
Two weeks after a single injection of suramin, the secretory and post-secretory ameloblasts of the rat incisor were filled with large lysosome-like vacuoles. At the light-microscope level, these vacuoles were positively stained with Alcian blue when MgCl2 was used at a critical electrolyte concentration varying between 0.1 and 0.3 M, whereas no staining appeared when MgCl2 varied between 0.7 and 0.9 M. Hyaluronidase digestion markedly reduced but did not totally abolish the staining, indicating that glycosaminoglycans were accumulated inside these vacuoles. Examination of these cells with the electron microscope revealed a polymorphic population of large vesicles, filled to various degrees with cetylpyridinium chloride (CPC)-positive and malachite green aldehyde (MGA)-positive material. The same pattern was observed in secretory odontoblasts but to a lesser extent. In the extracellular matrix, suramin-induced alterations appeared as large defects occurring during enamel formation. In predentin and dentin, the number and/or size of electron-dense aggregates resulting from CPC and MGA fixation, were enhanced in the suramin-injected rats. These aggregates were largely reduced or suppressed respectively by hyaluronidase digestion and chloroform/methanol treatment of the sections. The accumulation of glycosaminoglycans and phospholipids reported here inside ameloblasts and odontoblasts and in predentin and dentin supports the occurrence of suramin-induced mucopolysaccharidosis and lipidosis in this experimental animal model.
Similar content being viewed by others
References
Bancroft JD, Stevens A (1990) Theory and practice of histological techniques, 3rd edn. Churchill Livingstone, London
Bienkovsky RS (1983) Intracellular degradation of newly synthesized secretory proteins. Biochem J 214:1–10
Blumenthal NC (1981) Mechanism of proteoglycan inhibition of hydroxyapatite formation. In: Veis A (ed) The chemistry and biology of mineralized connective tissues. Elsevier/North Holland. New York, pp 509–515
Blumenthal NC, Posner AS, Silverman LD, Rosenberg IC (1979) Effects of proteoglycans on in vitro hydroxyapatite formation. Calcif Tissue Int 27:75–82
Boskey AL, Dick BL (1991) The effect of phosphatidylserine on in vitro hydroxyapatite growth and proliferation. Calcif Tissue Int 49:193–196
Buys CHC, Bouma JMW, Gruber M, Wisse E (1978) Induction of lysosomal storage by suramin. Naunyn-Schmiedeberg's Arch Pharmacol 304:183–190
Chardin H, Septier D, Goldberg M (1990) Visualization of glycosaminoglycans in rat incisor predentin and dentin with cetylpyridinium chloride-glutaraldehyde as fixative. J Histochem Cytochem 38:885–894
Christensen B, Lüllman-Rauch R (1988) On the alcianophilia of the drug suramin used as a tool for inducing experimental mucopolysaccharidosis. Histochemistry 89:365–367
Clark RD, Smith JG, Davidson EA (1965) Hexosamine and acidic glycosaminoglycans in human teeth. Biochim Biophys Acta 101:267–272
Coffey R, Leof E, Shipley G (1987) Suramin inhibition of growth factor receptor binding and mitogenicity in AKR-2B cells. J Cell Physiol 132:143–148
Constantopoulos G, Rees S, Cragg BG, Barranger JA, Brady RO (1980) Experimental model for mucopolysaccharidosis: Suramin-induced glycosaminoglycan and sphingomyelin accumulation in the rat. Proc Natl Acad Sci USA 77:3700–3704
Constantopoulos G, Rees S, Cragg BG, Barranger JA, Brady RO (1981) Effects of suramin on the activities of degradative enzymes of spingolipids in the rat. Res Commun Chem Pathol Pharmacol 32:87–97
Dirksen TR, Marinetti GV (1970) Lipids of bovine enamel and dentin and human bone. Calcif Tissue Res 6:1–10
Ellingson JS, Smith M, Larson LR (1977) Phospholipid composition and fatty acid profiles in bovine predentin. Calcif Tissue Res 24:127–133
Garret J, Coughlin S, Niman H (1984) Blockade of autocrine stimulation in simian sarcoma virus transformed cells reverses down-regulation of platelet-derived growth factor receptors. Proc Natl Acad Sci USA 81:7466–7470
Gibson DW, Duke BOC, Connor DH (1977) Histopathological studies on suramin toxicity in a chimpanzee. Trop Med Parasitol 28:387–405
Goldberg M, Escaig F (1987) Rapid freezing and malachite greenacrolein-osmium tetroxide freze-substitution fixation improve visualization of extracellular lipids in rat incisor predentin and dentin. J Histochem Cytochem 35:427–433
Goldberg M, Septier D (1983) Electron microscopic visualization of proteoglycans in rat incisor predentin and dentin with cuprolinic blue. Archs Oral Biol 28:79–83
Goldberg M, Septier D (1985) Improved lipid preservation by malachite green-glutaraldehyde fixation in rat incisor predentine and dentine. Archs Oral Biol 30:717–726
Goldberg M, Genotelle-Septier D, Weill R (1978) Glycoprotéines et protéoglycans de la matrice prédentinaire et dentinaire chez le rat: une étude ultrastructurelle. J Biol Buccale 6:75–90
Goldberg M, Septier D, Escaig-Haye F (1987) Glycoconjugates in dentinogenesis and dentine. Prog Histochem Cytochem 17:1–113
Hart DP, Young MR (1975) Interference with normal phagosomelysosome fusion in macrophages, using ingested yeast cells and suramin. Nature 256:47–49
Hjerpe A, Antonopoulos CA, Engfeldt B, Wikström B (1983) Analysis of dentine glycosaminoglycans using high-performance liquid chromatography. Calcif Tissue Int 35:496–501
Hooghwinkel GJM, Smits G (1957) The specificity of periodic acid-Schiff technique studied by a quantitative test-tube method. J Histochem Cytochem 5:120–126
Hunter G (1991) Role of proteoglycans in the provisional calcification of cartilage. A review and reinterpretation. Clin Orthoped 262:256–280
Idelman S (1963) Action du methanol-chloroforme sur les liposomes des cellules cortico-surrénales. C R Acad Sci (D) 282: 933–934
Katchburian E, Holt SJ (1968) Ultrastructural studies on lysosomes and acid phosphatase in odontoblasts. In: Symons NBB (ed) Dentine and pulp: their structure and reactions. Livingstone, Edinburgh, pp 43–57
Kielan MC, Steinman RM, Cohn ZA (1982) Intralysosomal accumulation of polyanions. I. Fusion of pinocytic and phagocytic vacuoles with secondary lysosomes. J Cell Biol 93:866–874
Kyle JW, Birkenmeier EH, Gwynn B, Voegler C, Hoppe PC, Hoffmann JW, Sly WS (1990) Correction of murine mucopolysaccharidosis VII by a human β-glucuronidase transgene. Proc Natl Acad Sci USA 87:3914–3918
Linde A (1984) Noncollagenous proteins and proteoglycans in dentinogenesis. In: Linde A (ed) Dentin and dentinogenesis, vol II. CRC Press, Boca Raton, pp 55–92
Linde A (1989) Dentin matrix proteins: Composition and possible functions in calcification. Anat Rec 224:154–166
Linde A, Persliden B (1977). Cathepsin D activity in isolated odontoblasts. Calcif Tiss Res 23:33–38
Linde A, Bhown M, Butler WT (1980) Non-collagenous proteins in dentin. A reexamination of proteins from rat incisor dentin utilizing techniques to avoid artifacts. J Biol Chem 255:5931–5942
Linde A, Lussi A, Crenshaw MA (1988) Mineral induction by immobilized polyanionic proteins. Calcif Tissue Int 44:286–295
Maroudas A (1975) Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology 12:233–240
McKusick VA, Neufeld EF (1983) The mucopolysaccharide storage diseases. In: Stanbury JB, Wyngaarden JB, Fredrickson DS, Goldstein JL, Brown MS (eds) The metabolic basis of inherited diseases. McGraw Hill, London, pp 751–777
Monneron A, Bernhard W (1966) Action de certaines enzymes sur des tissues inclus en Epon. J Microsc 5:697–714
Moriyama Y, Nelson N (1988) Inhibition of vacuolar H+-ATPases by fusidic acid and suramin. FEBS Lett 234:368–383
Nagai N (1979) Ultrastructural localization of acid phosphatase in odontoblasts of young rat incisors. Bull Tokyo Dent Coll 11:85–120
Nanci A, Bendayan M, Slavkin HC (1985) Enamel protein biosynthesis and secretion in mouse incisor secretory ameloblasts as revealed by high-resolution immunocytochemistry. J Histochem Cytochem 33:1153–1160
Neufeld EF (1991) Lysosomal storage diseases. Annu Rev Biochem 60:257–280
Nicholson WAP, Ashton BA, Höhling HJ, Quint P, Schreiber J (1977) Electron microprobe investigations into the process of hard tissue formation. Cell Tissue Res 177:331–345
Nygren H, Persliden B, Hansson H-A, Linde A (1979) Cathepsin D: Ultra-immunohistochemical localization in dentinogenesis. Calcif Tissue Int 29:251–252
Odutuga AA, Prout RES, Hoare RJ (1975) Hydroxyapatite precipitation in vitro by lipids extracted from mammalian hard and soft tissues. Arch Oral Biol 20:311–316
Prout RES, Odutuga AA, Tring FC (1973) Lipid analysis in enamel and dentin. Arch Oral Biol 18:373–380
Rahemtulla F, Prince CW, Butler WT (1984) Isolation and partial characterization of proteoglycans from rat incisors. Biochem J 218:877–885
Scott JE (1972) Histochemistry of Alcian blue. III. The molecular biological basis of staining by Alcian blue 8GX and analogous phthalocyanins. Histochemie 32:191–212
Scott JE (1973) Affinity, competition and specific interactions in the biochemistry and histochemistry of polyelectrolytes. Biochem Soc Trans 1:787–806
Scott JE, Dorling J (1965) Differential staining of acid glycosaminoglycans (mucopolysaccharides) by Alcian blue in salt solutions. Histochemie 5:221–223
Scott JE, Harbinson RJ (1968) Periodate oxidation of acidic polysaccharides. Inhibition by the electrostatic field of the substrate. Histochemie 14:215–220
Scott JE, Hughes EW (1983) Differential staining of polyanions according to critical electrolyte concentration principles in mixed solvents. J Microsc 129:209–219
Shapiro IM, Wuthier RE, Irving JT (1966) A study of the phospholipids of bovine dental tissues. I. Enamel and dentin. Arch Oral Biol 11:501–512
Shepard N, Mitchell N (1976) Simultaneous localization of proteoglycan by light and electron microscopy using toluidine blue O. A study of epiphyseal cartilage. J Histochem Cytochem 24:621–629
Shepard N, Mitchell N (1981) Acridine orange stabilization of glycosaminoglycans in beginning endochondral ossification. A comparative light and electron microscopic study. Histochemistry 70:107–114
Shimizu M, Tanabe T, Fukae M (1979) Proteolytic enzyme in porcine immature enamel. J Dent Res 58(B):782–789
Smith CE (1979) Ameloblasts secretory and resorptive function. J Dent Res 58(B):695–706
Sundström B (1971) New aspects on the utilization of inorganic sulphate during dentin formation. Histochemie 26:61–66
Takagi M, Parmley RT, Deny FR (1981) Ultrastructural localization of complex carbohydrates in odontoblasts, predentin and dentin. J Histochem Cytochem 29:747–758
Tas J, Mendelson D, Noorden CJF (1983) Cuprolinic blue: a specific dye for single-stranded RNA in the presence of magnesium chloride. I. Fundamental aspects. Histochem J 15:801–814
Teichman RJ, Fujimoto M, Yanagimachi R (1972) A previously unrecognized material in mammalian spermatozoa as revealed by malachite green and pyronine. Biol Reprod 7:73–81
Teichman RJ, Cummins JM, Takei GH (1974) The characterization of a malachite green stainable glutaraldehyde extractable phospholipid in rabbit spermatozoa. Biol Reprod 10:565–577
Tenenbaum HC, Hunter GK (1987) Chondroitin sulfate inhibits calcification of bone formed in vitro. Bone Miner 2:43–51
Williams G, Jackson DS (1956) Two organic fixatives for acid mucopolysaccharides. Stain Technol 31:189–191
Wuthier RE (1984) Lipids in dentinogenesis. In: Linde A (ed) Dentin and dentinogenesis, vol. II. CRC Press, Boca Raton, pp 93–106
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gritli, A., Septier, D. & Goldberg, M. Suramin-induced mucopolysaccharidosis in rat incisor. Cell Tissue Res 273, 53–64 (1993). https://doi.org/10.1007/BF00304611
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00304611