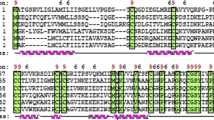
Abstract
The Shrunken-2 (Sh2) and Brittle-2 (Bt2) genes of maize encode subunits of the tetrameric maize endosperm ADPglucose pyrophosphorylase. However in all sh2 and bt2 mutants so far examined, measurable ADPglucose pyrophosphorylase activity remains. We have investigated the origin of the residual activity found in various sh2 and bt2 mutants as well as tissue specific expression and post-translational modification of the Sh2 and Bt2 proteins. Sh2 and Bt2 cDNAs were expressed in Escherichia coli and antibodies were prepared against the resulting proteins. SH2 and BT2 specific antibodies were used to demonstrate that SH2 and BT2 are endosperm specific, are altered or missing in various sh2 or bt2 mutants, and have a mol. wt. of 54 and 51 kDa respectively in the wild type. The Sh2 and Bt2 transcripts are also endosperm specific. Ten sh2 and eight bt2 mutants show varying severity of phenotypes expressed at transcript, protein subunit and kernel level. Synthesis of multiple transcripts and proteins commonly occurs as a result of sh2 or bt2 mutation. While all mutants produce detectable enzymic activity, not all produce detectable transcripts and proteins. To examine the origin of the apparent non-SH2/BT2 endosperm enzymic activity, homologs of Sh2 and Bt2, designated Agp1 and Agp2 respectively, were isolated from an embryo cDNA library and found to hybridize to endosperm transcripts distinct from those of Sh2 and Bt2. Thus Agp1 and Agp2 or closely related genes may be responsible for the residual activity in some sh2 and bt2 mutants. Surprisingly, no evidence of post-translational modification of the SH2 and BT2 protein subunits was detected.
Similar content being viewed by others
References
Anderson DJ, Blobel G (1983) Immunoprecipitation of proteins from cell free translations. Meth Enzymol 96:111–120
Anderson JM, Hnilo J, Larson R, Okita TW, Morell M, Preiss J (1989) The encoded primary sequence of a rice seed ADP-glucose pyrophosphorylase subunit and its homology to the bacterial enzyme. J Biol Chem 264:12238–12242
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1988) Current protocols in molecular biology. Greene Publishing Associates — Wiley Interscience, New York
Bae JM, Giroux M, Hannah L (1990) Cloning and characterization of the brittle-2 gene of maize. Maydica 35:317–322
Bhave MR, Lawrence S, Barton C, Hannah LC (1990) Identification and molecular characterization of shrunken-2 cDNA clones of maize. Plant Cell 2:581–588
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Bryce WH, Nelson OE (1979) Starch-synthesizing enzymes in the endosperm and pollen of maize. Plant Physiol 63:312–317
Burr B, Burr FA, Matz EC (1991) Database for loci mapped in TxCM and CoxTx RI families. Maize Genetics Coop Newslett 65:105–110
Cameron JW, Teas HJ (1954) Carbohydrate relationships in developing and mature endosperms of brittle and related maize genotypes. Am J Bot 41:50–55
Chourey PS, Nelson OE (1976) The enzymatic deficiency conditioned by the shrunken-1 mutations in maize. Biochem Genet 14:1041–1055
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81:1991–1995
Dang PL, Boyer CD (1989) Comparison of soluble starch synthases and branching enzymes from leaves and kernels of normal and amylose-extender maize. Biochem Genet 27:521–532
Dickinson DB, Preiss J (1969) Presence of ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize endosperm. Plant Physiol 44:1058–1062
Hannah LC, Nelson OE Jr (1975) Characterization of adenosine diphosphate glucose pyrophosphorylases from developing maize seeds. Plant Physiol 55:297–302
Hannah LC, Nelson OE Jr (1976) Characterization of ADP-glucose pyrophosphorylase from shrunken-2 and brittle-2 mutants of maize. Biochem Genet 14:547–560
Hannah LC, Tuschall DM, Mans RJ (1980) Multiple forms of maize endosperm ADP-glucose pyrophosphorylase and their control by shrunken-2 and brittle-2. Genetics 95:961–970
Hatzfeld WD, Stitt M (1990) A study of the rate of recycling of triose phosphates in heterotrophic Chenopodium rubrum cells, potato tubers, and maize endosperm. Planta 180:198–204
Heijne G von, Steppuhn J, Herrmann RG (1989) Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem 180:535–545
Kim WT, Franceschi VR, Okita TW, Robinson NL, Morell M, Preiss J (1989) Immunocytochemical localization of ADPglucose pyrophosphorylase in developing potato tuber cells. Plant Physiol 91:217–220
Krishnan HB, Reeves CD, Okita TW (1986) ADPglucose pyrophosphorylase is encoded by different mRNA transcripts in leaf and endosperm of cereals. Plant Physiol 81:642–645
Laemmli V (1970) Cleavage of structural proteins during the assembly of the bacteriophage T4. Nature 227:680–685
Lin TP, Caspar T, Somerville CR, Preiss J (1988) Starch deficient mutant of Arabidopsis thaliana with low ADPglucose pyrophosphorylase activity lacks one of the two subunits of the enzyme. Plant Physiol 88:1175–1181
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
McCarty DR (1986) A simple method for extraction of RNA from maize tissues. Maize Genet Coop Newslett 60:61
McCarty DR, Shaw JR, Hannah LC (1986) The cloning, genetic mapping, and expression of the constitutive sucrose synthase locus of maize. Proc Natl Acad Sci USA 83:9099–9103
McCarty DR, Hattori T, Carson CB, Vasil V, Lazar M, Vasil IK (1991) The Viviparous-1 developmental gene of maize encodes a novel transcriptional activator. Cell 66:895–905
Morell MK, Bloom M, Knowles V, Preiss J (1987) Subunit structure of spinach leaf ADPglucose pyrophosphorylase. Plant Physiol 85:182–187
Nakata PA, Greene TW, Anderson JM, Smith-White BJ, Okita TW, Preiss J (1991) Comparison of the primary sequences of two potato tuber ADP-glucose pyrophosphorylase subunits. Plant Mol Biol 17:1089–1093
Nelson OE Jr, Burr B (1973) Biochemical genetics of higher plants. Annu Rev Plant Physiol 24:493–518
Nelson OE, Rines HW (1962) The enzymatic deficiency in the waxy mutant of maize. Biochem Biophys Res Commun 9:297–300
Okita TW, Nakata PA, Anderson JM, Sowokinos J, Morell M, Preiss J (1990) The subunit structure of potato tuber ADPglucose pyrophosphorylase. Plant Physiol 93:785–790
Pozueto-Romero J, Frehner M, Viale AM, Akazawa T (1991) Direct transport of ADPglucose by an adenylate translocator is linked to starch biosynthesis in amyloplasts. Proc Natl Acad Sci USA 88:5769–5773
Preiss J, Danner S, Summers PS, Morell M, Barton CR, Yang L, Nieder M (1990) Molecular characterization of the brittle-2 gene effect on maize endosperm ADPglucose pyrophosphorylase subunits. Plant Physiol 92:881–885
Preiss J, Ball K, Hutney J, Smith-White B, Li L, Okita TW (1991) Regulatory mechanisms involved in the biosynthesis of starch. Pure Appl Chem 63:535–544
Shaw JR, Hannah LC (1992) Genomic nucleotide sequence of a wild type Shrunken-2 allele of Zea mays. Plant Physiol 98:1214–1216
Smith-White BJ, Preiss J (1992) Comparison of proteins of ADPglucose pyrophosphorylase from diverse sources. J Mol Evol 34:449–464
Stark DM, Timmerman KP, Barry GF, Preiss J, Kishore GM (1992) Regulation of the amount of starch in plant tissues by ADP glucose pyrophosphorylase. Science 258:287–291
Studier FW, Rosenberg AH, Dunn JJ, Dubendorf JW (1990) Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol 185:60–89
Sullivan TD, Strelow LI, Illingworth CA, Phillips RL, Nelson OE Jr (1991) The maize brittle-1 locus: molecular characterization based on DNA clones isolated using the dSpm-tagged brittle-1-mutable allele. Plant Cell 3:1337–1348
Tsai CY, Nelson OE (1966) Starch deficient maize mutant lacking adenosine diphosphate glucose pyrophosphorylase activity. Science 151:341–343
Tuschall DM, Hannah LC (1982) Altered maize endosperm ADPglucose pyrophosphorylase from revertants of a shrunken-2 dissociation allele. Genetics 100:105–111
Viola R, Davies HV, Chudeck AR (1991) Pathways of starch and sucrose biosynthesis in developing tubers of potato (Solanum tuberosum L.) and seeds of faba bean (Vicia faba L.). Planta 183:202–208
Author information
Authors and Affiliations
Additional information
Communicated by H. Saedler
Rights and permissions
About this article
Cite this article
Giroux, M.J., Hannah, L.C. ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize. Molec. Gen. Genet. 243, 400–408 (1994). https://doi.org/10.1007/BF00280470
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00280470