Abstract
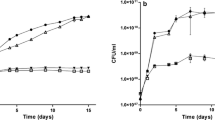
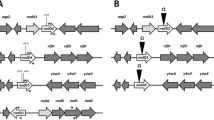
A 7.1 kb EcoRI fragment from Azospirillum brasilense, that hybridized with a probe carrying the ntrBC genes from Bradyrhizobium japonicum, was cloned. The nucleotide sequence of a 3.8 kb subfragment was established. This led to the identification of two open reading frames, encoding polypeptides of 401 and 481 amino acids, that were similar to NtrB and NtrC, respectively. A broad host range plasmid containing the putative Azospirillum ntrC gene was shown to restore nitrogen fixation under free-living conditions to a ntrC-Tn5 mutant of Azorhizobium caulinodans. Several Tn5 insertion mutants were isolated in the ntrBC coding region in A. brasilense. These mutants were prototrophic and Nif+. However, their nitrogenase activity was slightly lower than in the wild type and they were unable to grow on nitrate as sole nitrogen source. Under microaerobiosis and in the absence of ammonia, a nifA-lacZ fusion was expressed in the mutants at about 60% of the level in the wild type. In the presence of ammonia, the fusion was similarly expressed (60% of the maximum) both in the wild type and mutants. Addition of ammonia to a nitrogen-fixing culture of ntrBC mutants did not abolish nitrogenase activity, in contrast with the wild type. It thus appears that in Azospirillum the ntrBC genes are not essential for nitrogen fixation, although NtrC controls nifA expression to some extent. They are, however, required for the switch-off of nitrogenase activity.
Similar content being viewed by others
References
Allen LL, Hanson RS (1985) Construction of broad host range cosmid cloning vectors: identification of genes necessary for growth of Methylobacterium organophilum on methanol. J Bacteriol 161:955–962
Bozouklian H, Fogher C, Elmerich C (1986) Cloning and characterization of the glnA gene of Azospirillum brasilense Sp7. Ann Inst Pasteur Microbiol 137:3–18
Buikema WJ, Szeto WW, Lemley PV, Orme-Johnson WH, Ausubel FM (1985) Nitrogen fixation specific regulatory genes of Klebsiella pneumoniae and Rhizobium meliloti share homology with the general nitrogen regulatory gene ntrC of K. pneumoniae. Nucleic Acids Res 13:4539–4555
Ditta G, Virts E, Palomares A, Kim CH (1987) The nifA gene of Rhizobium meliloti is oxygen regulated. J Bacteriol 169:3217–3223
Elmerich C (1991) Genetics and regulation of Mo-nitrogenase. In: Dilworth MJ, Glenn AR (eds) Biology and Biochemistry of Nitrogen Fixation. Elsevier, Amsterdam, pp 103–141
Elmerich C, Bozouklian H, Vieille C, Fogher C, Perroud B, Perrin A, Vanderleyden J (1987) Azospirillum: genetics of nitrogen fixation and interaction with plants. Philos Trans R Soc Lond [Biol] 317:183–192
Elmerich C, Zimmer W, Vieille C (1992) Associative nitrogen-fixing bacteria. In: Stacey G, Burris RH, Evans HJ (eds) Biological Nitrogen Fixation. Chapman and Hall, New York, pp 212–258
Fani R, Allotta G, Bazzicalupo M, Ricci F, Schipani C, Polsinelli M (1989) Nucleotide sequence of the gene encoding the nitrogen iron protein (nifH) of Azospirillum brasilense and identification of a region controlling nifH transcription. Mol Gen Genet 220:81–87
Friedman AM, Long SR, Brown SE, Buikema WJ, Ausubel FM (1982) Construction of a broad host range cosmid cloning vector and its use in genetic analysis of Rhizobium mutants. Gene 18:289–296
Galimand M, Perroud B, Delorme F, Paquelin A, Vieille C, Bozouklian H, Elmerich C (1989) Identification of DNA regions homologous to nitrogen fixation genes nifE, nifUS and fixABC in Azospirillum brasilense Sp7. J Gen Microbiol 135:1047–1059
Henikoff S (1984) Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene 28:351–359
Jones R, Haselkorn R (1989) The DNA sequence of the Rhodobacter capsulatus ntrA, ntrB and ntrC gene analogues required for nitrogen fixation. Mol Gen Genet 215:507–516
Kaminski PA, Elmerich C (1991) Involvement of fixLJ in the regulation of nitrogen fixation in Azorhizobium caulinodans. Mol Microbiol 5:665–673
Kokotek W, Lotz W (1989) Construction of a lacZ-kanamycin-resistance cassette, useful for site-directed mutagenesis and as a promoter probe. Gene 84:467–471
Liang YY, Kaminski PA, Elmerich C (1991a) Identification of a nifA-like regulatory gene of Azospirillum brasilense Sp7 expressed under conditions of nitrogen fixation and in the presence of air and ammonia. Mol Microbiol 5:2735–2744
Liang J, Nielsen GM, Lies DP, Burris RH, Roberts GP, Ludden PW (1991b) Mutations in the draT and draG genes of Rhodospirillum rubrum result in loss of regulation of nitrogenase by reversible ADP-ribosylation. J Bacteriol 173:6903–6909
Liang YY, de Zamaroczy M, Arsène F, Paquelin A, Elmerich C (1992) Regulation of nitrogen fixation genes in Azospirillum brasilense Sp7: involvement of the nifA, glnB and glnA gene products. FEMS Microbiol Lett 100:113–120
Ludden PW, Roberts GP (1989) Regulation of nitrogenase activity by reversible ADP-ribosylation. Curr Top Cell Regul 30:23–55
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Martin GB, Chapman KA, Barry KC (1988) Role of the Brady-rhizobium japonicum ntrC gene product in differential regulation of the glutamine synthetase II gene (glnII). J Bacteriol 170:5452–5459
McFarlane SA, Merrick MJ (1985) The nucleotide sequence of the nitrogen regulatory gene (ntrB), and the glnA-ntrBC intergenic region of Klebsiella pneumoniae. Nucleic Acids Res 13:7591–7606
Merrick MJ (1988) Regulation of nitrogen assimilation by bacteria. In: Code JA, Ferguson SJ (eds) The nitrogen and sulphur cyles. Cambridge University Press, Cambridge, pp 331–361
Merrick MJ (1992) Regulation of nitrogen fixation genes in freeliving and symbiotic bacteria. In: Stacey G, Burris RH, Evans H (eds) Biological nitrogen fixation. Chapman and Hall, New York, pp 835–876
Messing J (1983) New M13 vectors for cloning. Methods Enzymol 101:20–78
Miller JH (1972) Assay of β-galactosidase. In: Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York pp 352–355
Nixon BT, Ronson CW, Ausubel FM (1986) Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc Natl Acad Sci USA 83:7850–7854
Pawlowski K, Ratet P, Schell J, de Bruijn FJ (1987) Cloning and characterization of nifA and ntrC genes of the stem nodulating bacterium ORS571, the nitrogen fixing symbiont of Sesbania rostrata: regulation of nitrogen fixation (nif) genes in the free-living versus symbiotic state. Mol Gen Genet 206:207–219
Pedrosa FO, Yates MG (1984) Regulation of nitrogen fixation (nif) genes of Azospirillum brasilense by nifA and ntr (gln) type gene products. FEMS Microbiol Lett 23:95–101
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology 1:784–791
Staden R (1983) Computer methods for DNA sequencers. In: Hindley J, Worth TS, Bividon RH (eds) DNA sequencing. Elsevier Biochemical Press, Amsterdam, pp 311–368
Stock JB, Ninfa AJ, Stock AM (1989) Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev 53:450–490
Stocker K, Reijnders WNM, Oltman LF, Stouthamer AH (1989) Initial cloning and sequencing of hydHG, an operon homologous to ntrBC and regulating the labile hydrogenase activity in Escherichia coli K-12. J Bacteriol 171:4448–4456
Szeto WW, Nixon T, Ronson CW, Ausubel FM (1987) Identification and characterization of the Rhizobium meliloti ntrC gene: R. meliloti has separate regulatory pathways for activating nitrogen fixation genes in free-living and in symbiotic cells. J Bacteriol 169:1423–1432
Tarrand JJ, Krieg NR, Döbereiner J (1978) A taxonomic study of the Spirillum lipoferum group, with description of a new genus, Azospirillum gen. nov. and two species Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol 24:967–980
Toukdarian A, Kennedy C (1986) Regulation of nitrogen metabolism in Azotobacter vinelandii: isolation of ntr and glnA genes and construction of ntr mutants. EMBO J 5:399–407
Turbanti L, Bazzicalupo M, Casalone E, Fani R, Gallori E, Polsinelli M (1988) Mutants of Azospirillum resistant to methylammonium. Arch Microbiol 150:421–425
Weiss V, Magasanik B (1988) Phosphorylation of nitrogen regulator I (NRI) of Escherichia coli. Proc Natl Acad Sci USA 85:8919–8923
de Zamaroczy M, Delorme F, Elmerich C (1989) Regulation of transcription and promoter mapping of the structural genes for nitrogenase (nifHDK) of Azospirillum brasilense Sp7. Mol Gen Genet 220:88–94
de Zamaroczy M, Delorme F. Elmerich C (1990) Characterization of three different nitrogen-regulated promoter regions for the expression of glnB and glnA in Azospirillum brasilense Sp7. Mol Gen Genet 224:421–430
de Zamaroczy M, Paquelin A, Elmerich C (1993) Functional organization of the glnB-glnA cluster of Azospirillum brasilense. J Bacteriol 175:2507–2515
Zhang Y, Burris RH, Roberts GP (1992) Cloning, sequencing, mutagenesis and functional characterization of draT and draG genes from Azospirillum brasilense. J Bacteriol 174:3364–3369
Author information
Authors and Affiliations
Additional information
Communicated by H. Hennecke
Rights and permissions
About this article
Cite this article
Liang, Y.Y., Arsène, F. & Elmerich, C. Characterization of the ntrBC genes of Azospirillam brasilense Sp7: Their involvement in the regulation of nitrogenase synthesis and activity. Molec. Gen. Genet. 240, 188–196 (1993). https://doi.org/10.1007/BF00277056
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00277056