Summary
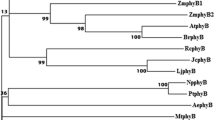
Southern blot analysis indicates that the rice genome contains single copies of genes encoding type A (phyA) and type B (phyB) phytochromes. We have isolated overlapping cDNA and genomic clones encoding the entire phyB polypeptide. This monocot sequence is more closely related to phyB from the dicot, Arabidopsis (73% amino acid sequence identity), than it is to the phyA gene in the rice genome (50% identity). These data support the proposal that phyA and phyB subfamilies diverged early in plant evolution and that subsequent divergence accompanied the evolution of monocots and dicots. Moreover, since rice and Arabidopsis phyB polypeptides are more closely related to one another (73% identity) than are monocot and dicot phyA sequences (63–65% identity), it appears that phyB has evolved more slowly than phyA. Sequence conservation between phyA and phyB is greatest in a central core region surrounding the chromophore attachment site, and least toward the amino-terminal and carboxy-terminal ends of the polypeptides, although hydropathy analysis suggests that the overall structure of the two phytochromes has been conserved. Gene-specific Northern blot analysis indicates that, whereas phyA is negatively regulated by phytochrome in rice seedling shoots in the manner typical of monocots, phyB is constitutively expressed irrespective of light treatment. In consequence, phyA and phyB transcripts are equally abundant in fully green tissue. Since Arabidopsis phyB mRNA levels are also unaffected by light, the present results suggest that this mode of regulation is evolutionarily conserved among phyB genes, perhaps reflecting differences in the functional roles of the different phytochrome subfamilies.
Similar content being viewed by others
References
Abe H, Yamamoto KT, Nagatani A, Furuya M (1985) Characterization of green tissue-specific phytochrome isolated immunochemically from pea seedlings. Plant Cell Physiol 326:1387–1399
Briggs WR, Chon HP (1966) The physiological versus spectrophotometric status of phytochrome in corn coleoptiles. Plant Physiol 41:1159–1166
Brockmann J, Schäfer E (1982) Analysis of Pfr destruction in Amaranthus caudatus L.: Evidence for two pools of phytochrome. Photochem Photobiol 35:555–558
Bruce WB, Christensen AH, Klein T, Fromm M, Quail PH (1989) Photoregulation of a phytochrome gene promoter from oat transferred into rice by particle bombardment. Proc Natl Acad Sci USA 86:9692–9696
Christensen AH, Quail PH (1989) Structure and expression of a maize phytochrome-encoding gene. Gene 85:381–390
Colbert JT, Hershey HP, Quail PH (1983) Autoregulatory control of translatable phytochrome mRNA levels. Proc Natl Acad Sci USA 80:2248–2252
Colbert JT, Hershey HP, Quail PH (1985) Phytochrome regulation of phytochrome mRNA abundance. Plant Mot Biol 5:91–102
Cordonnier M-M, Greppin H, Pratt L (1986) Phytochrome from green Avena shoots characterized with a monoclonal antibody to phytochrome from etiolated Pisum shoots. Biochemistry 25:7657–7666
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12:387–395
Frohman MA, Dush MK, Martin GR (1988) Rapid production of full length cDNA from rare transcripts: Amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA 85:8998–9002
Furuya M (1987) Phytochrome and Photoregulation in Plants. Proceedings of the Yamada Conference XVI, Academic Press, New York Tokyo
Hershey HP, Colbert JT, Lissemore JL, Barker RF, Quail PH (1984) Molecular cloning of cDNA for Avena phytochrome. Proc Natl Acad Sci USA 81:2332–2336
Hershey HP, Barker RE, Idler KB, Lissemore JL, Quail PH (1985) Analysis of cloned cDNA and genomic sequences for phytochrome: Complete amino acid sequence for two gene products expressed in etiolated Avena. Nucleic Acids Res 13:8543–8559
Hillman WS (1967) The physiology of phytochrome. Annu Rev Plant Physiol 18:301–324
Hilton JR, Thomas B (1987) Photoregulation of phytochrome synthesis in germinating embryos of Avena sativa L. J Exp Bot 38:1704–1712
Jabben M, Deitzer GF (1978) Spectrophotometric phytochrome measurements in light-grown Arena sativa L. Planta 143:309–313
Jabben M, Holmes MG (1983) Phytochrome in light-grown plants. In: Shropshire W, Mohr H (eds) Photomorphogenesis. Springer-Verlag, Berlin; pp 704–722
Kay SA, Keith B, Shinozaki K, Chua N-H (1989a) The sequence of the rice phytochrome gene. Nucleic Acids Res 17:2865–2866
Kay SA, Keith B, Shinozaki K, Chye M-L, Chua N-H (1989b) The rice phytochrome gene: Structure, autoregulated expression, and binding of GT-1 to a conserved site in the 5′ upstream region. Plant Cell 1:351–360
Konomi K, Abe H, Furuya M (1987) Changes in the content of phytochrome I and II apoproteins in embryonic axes of pea seeds during imibition. Plant Cell Physiol 28:1443–1451
Kronenberg GHM, Kendrick RE (1986) Phytochrome: The physiology of action. In: Kendrick RE, Kronenberg GHM (eds) Photomorphogenesis in Plants. Martinus Nijhoff, Dordrecht, pp 99–114
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Lissemore JL, Quail PH (1988) Rapid transcriptional regulation by phytochrome of the genes for phytochrome and chlorophyll a/b-binding protein in Avena sativa. Mol Cell Biol 8:4840–4850
Lissemore JL, Colbert JT, Quail PH (1987) Cloning of cDNA for phytochrome from etiolated Cucurbita and coordinate photoregulation of the abundance of two distinct phytochrome transcripts. Plant Mol Biol 8:485–496
Martin WF, Gierl A, Saedler H (1989) Molecular evidence for pre-Cretaceous angiosperm origins. Nature 339:46–48
Maxam AM, Gilbert W (1980) Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol 65:499–560
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Pratt LH, Cordonnier M-M (1987) Phytochrome from green Avena. In: Furuya M (ed) Phytochrome and Photoregulation in Plants. Academic Press, Tokyo, pp 83–94
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Sato N (1988) Nucleotide sequence and expression of the phytochrome gene in Pisum sativum: Differential regulation by light of multiple transcripts. Plant Mol Biol 11:697–710
Sharrock RA, Quail PH (1989) Novel phytochrome sequences in Arabidopsis thaliana: Structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev 3:1745–1757
Sharrock RA, Lissemore JL, Quail PH (1986) Nucleotide and derived amino acid sequence of a Cucurbita phytochrome cDNA clone: Identification of conserved features by comparison with Avena phytochrome. Gene 47:287–295
Sharrock RA, Parks BM, Koornneef M, Quail PH (1988) Molecular analysis of the phytochrome deficiency in an Aurea mutant of tomato. Mol Gen Genet 213:9–14
Shimazaki Y, Pratt LH (1985) Immunochemical detection with rabbit polyclonal and mouse monoclonal antibodies of different pools of phytochrome from etiolated and green Avena shoots. Planta 164:333–344
Shimazaki Y, Pratt LH (1986) Immunoprecipitation of phytochrome from green Avena by rabbit antisera to phytochrome from etiolated Avena. Planta 168:512–515
Shimazaki Y, Cordonnier M-M, Pratt LH (1983) Phytochrome quantitation in crude extracts of Avena by enzyme-linked immunosorbent assay with monoclonal antibodies. Planta 159:534–544
Shropshire W Jr, Mohr H (eds) (1983) Photomorphogenesis. Encyclopedia of Plant Physiology, New Series, vols 16A, 16B. Springer-Verlag, Heidelberg Berlin New York Tokyo
Tokuhisa JG, Quail PH (1983) Spectral and immunochemical characterization of phytochrome isolated from light-grown Avena sativa (Abstract). Plant Physiol (Suppl) 72:85
Tokuhisa JG, Quail PH (1987) The levels of two distinct species of phytochrome are regulated differently during germination in Avena. Planta 172:371–377
Tokuhisa JG, Quail PH (1989) Phytochrome in green tissue: Partial purification and characterization of the 118-kilo Dalton phytochrome species from light-grown Avena sativa L. Photochem Photobiol 50:143–152
Tokuhisa JG, Daniels SM, Quail PH (1985) Phytochrome in green tissue: Spectral and immunochemical evidence for two distinct molecular species of phytochrome in light grown Avena sativa. Planta 164:321–332
Vierstra RD, Quail PH (1986) Phytochrome: The Protein: In: Kendrick RE, Kronenberg GHM (eds) Photomorphogenesis in Plants. Martinus Nijhoff, Dordrecht, pp 35–60
Author information
Authors and Affiliations
Additional information
Communicated by R.G. Herrmann
Rights and permissions
About this article
Cite this article
Dehesh, K., Tepperman, J., Christensen, A.H. et al. phyB is evolutionarily conserved and constitutively expressed in rice seedling shoots. Molec. Gen. Genet. 225, 305–313 (1991). https://doi.org/10.1007/BF00269863
Issue Date:
DOI: https://doi.org/10.1007/BF00269863