Summary
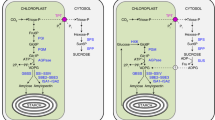
The key regulatory step in starch biosynthesis is catalyzed by the tetrameric enzyme ADP-glucose pyrophosphorylase (AGPase). In leaf and storage tissue, the enzyme catalyzes the synthesis of ADP-glucose from glucose-l-phosphate and ATP. Using heterologous probes from maize, two sets (B and S) of cDNA clones encoding potato AGPase were isolated from a tuber-specific cDNA library. Sequence analysis revealed homology to other plant and bacterial sequences. Transcript sizes are 1.9 kb (AGPase B) and 2.1 kb (AGPase S). Northern blot experiments show that the two genes differ in their expression patterns in different organs. Furthermore, one of the genes (AGPase S) is strongly inducible by metabolizable carbohydrates (e.g. sucrose) at the RNA level. The accumulation of AGPase S mRNA was always found to be accompanied by an increase in starch content. This suggests a link between aGPase S expression and the status of a tissue as either a sink for or a source of carbohydrates. By contrast, expression of AGPase B is much less variable under various experimental conditions.
Similar content being viewed by others
References
Amasino RM (1986) Acceleration of nucleic acid hybridisation rate by polyethylene glycol. Anal Biochem 152:304–307
Anderson JM, Hnilo J, Raymond L, Okita TW, Morell M, Preiss J (1989) The encoded primary sequence of a rice seed ADPglucose pyrophosphorylase subunit and its homology to the bacterial enzyme. J Biol Chem 264:12238–12242
Bae JM, Giroux M, Hannah LC (1990) Cloning and characterization of the brittle-2 gene of maize. Maydica, in press
Baecker PA, Furlong CE, Preiss J (1983) Biosynthesis of bacterial glycogen. Primary structure of Escherichia coli ADP-glucose synthetase as deduced from the nucleotide sequence of the glgC gene. J Biol Chem 258:5084–5088
Bhave MR, Lawrence S, Barton C, Hannah LC (1990) Identification and molecular characterization of shrunken-2 cDNA clones of maize. Plant Cell, in press
Dickinson DB, Preiss J (1969) Presence of ADPglucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize endosperm. Plant Physiol 44:1058–1062
Groussol J, Delrot S, Caruhel P, Bonnemain JL (1986) Design of an improved exudation method for phloem sap collection and its use for the study of phloem mobility of pesticides. Physiol Vég 24:123–133
Haugen TH, Ishaque A, Preiss J (1976) Biosynthesis of bacterial glycogen. Characterization of the subunit structure of Escherichia coli B glucose-1-phosphate adenylyltransferase. J Biol Chem 251:7880–7885
Hawker JS, Marschner H, Krauss A (1979) Starch synthesis in developing potato tubers. Physiol Plant 46:25–30
Ho LC (1988) Metabolism and compartmentation of imported sugars in sink organs in relation to sink strength. Annu Rev Plant Physiol Plant Mol Biol 39:355–378
Kauss H (1987) Some aspects of calcium-dependent regulation in plant metabolism. Annu Rev Plant Physiol 38:47–72
King RW, Zeevaart JAD (1974) Enhancement of phloem exudation from cut petioles by chelating agents. Plant Physiol 53:96–103
Krishnan HB, Reeves CD, Okita TW (1986) ADPglucose pyrophosphorylase is encoded by different mRNA transcripts in leaf and endosperm of cereals. Plant Physiol 81:642–645
Lehmann M, Preiss J (1980) Biosynthesis of bacterial glycogen: purification and properties of Salmonella typhimurium LT-2 adenosine diphosphate glucose pyrophosphorylase. J Bacteriol 143:120–127
Lin TP, Caspar T, Somerville C, Preiss J (1988a) Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) Heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiol 86:1131–1135
Lin TP, Caspar T, Somerville CR, Preiss J (1988b) A starch deficient mutant of Arabidopsis thaliana with low ADPglucose pyrophosphorylase activity lacks one of the two subunits of the enzyme. Plant Physiol 88:1175–1181
Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 163:21–26
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Moreland DE (1980) Mechanisms of action of herbicides. Annu Rev Plant Physiol 31:597–638
Morell MK, Bloom M, Knowles V, Preiss J (1987a) Subunit structure of spinach leaf ADP-glucose pyrophosphorylase. Plant Physiol 85:182–187
Morell M, Bloom M, Larsen R, Okita TW, Preiss J (1987b) Biochemistry and molecular biology of starch synthesis. In: Key JL, McIntosh L (eds) Plant gene systems and their biology. Alan Liss, New York, pp 227–242
Morell M, Bloom M, Preiss J (1988) Affinity labeling of the allosteric activator site(s) of spinach leaf ADP-glucose pyrophosphorylase. J Biol Chem 263:633–637
Olive MR, Ellis RJ, Schuch WW (1989) Isolation and nucleotide sequences of cDNA clones encoding ADPglucose pyrophosphorylase polypeptides from wheat leaf and endosperm. Plant Mol Biol 12:525–538
Plaxton W, Preiss J (1987) Purification and properties of nonproteolytic degraded ADPglucose pyrophosphorylase from maize endosperm. Plant Physiol 83:105–112
Preiss J (1978) Regulation of ADPglucose pyrophosphorylase. In: Meister A (ed) Advances in enzymology and related areas of molecular biology. John Wiley and Sons, New York, pp 317–381
Preiss J (1982a) Regulation of the biosynthesis and degradation of starch. Annu Rev Plant Physiol 33:431–454
Preiss J (1982b) Biosynthesis of starch and its regulation. In: Loewus FA, Tanner W (eds) Encyclopedia of plant physiology. New Series, vol 13A. Springer, Berlin Heidelberg, pp 397–417
Preiss J, Bloom M, Morell M, Knowles VL, Plaxton WC, Okita TW, Larsen R, Harmon AC, Putnam-Evans C (1987) Regulation of starch synthesis: enzymological and genetic studies. In: Bruening G, Harada J, Kosuge T (eds) Tailoring genes for crop improvement: an agricultural perspective. Plenum Press, New York, pp 133–152
Proudfoot N (1984) The end of the message and beyond. Nature 307:412–413
Proudfoot NJ, Brownlee GG (1976) 3′ Non-coding region sequences in eukaryotic messenger RNA. Nature 263:211–214
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Sowokinos JR (1976) Pyrophosphorylases in Solanum tuberosum. I. Changes in ADPglucose and UDPglucose pyrophosphorylase activities associated with starch biosynthesis during tuberization, maturation, and storage of potatoes. Plant Physiol 57:63–68
Sowokinos JR, Preiss J (1982) Pyrophosphorylases in Solanum tuberosum. III. Purification, physical and catalytic properties of ADPglucose pyrophosphorylase in potato. Plant Physiol 69:1459–1466
Tsai CY, Nelson OE (1966) Starch-deficient maize mutant lacking adenosine diphosphate glucose pyrophosphorylase activity. Science 151:341–343
Tully RE, Hanson AD (1979) Amino acids translocated from turgid and water-stressed barley leaves. Plant Physiol 64:460–466
Turgeon R (1989) The sink-source transition in leaves. Annu Rev Plant Physiol Plant Mol Biol 40:119–138
Weibull J, Ronquist F, Brishammar S (1990) Free amino acid composition of leaf exudates and phloem sap. Plant Physiol 92:222–226
Author information
Authors and Affiliations
Additional information
Communicated by J. Schell
Rights and permissions
About this article
Cite this article
Müller-Röber, B.T., Koßmann, J., Hannah, L.C. et al. One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Molec. Gen. Genet. 224, 136–146 (1990). https://doi.org/10.1007/BF00259460
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00259460