Summary
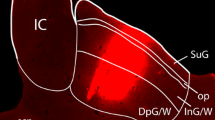
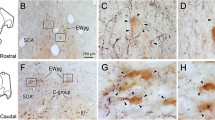
We have studied the serotonergic (5-HT) projection to the cat superior colliculus (SC) using serotonin antibody immunocytochemistry and retrograde transport of peroxidase-conjugated wheatgerm agglutinin (WGA-HRP). In 3 experiments, the two labels were combined in order to double label cells with both anti-5-HT and WGA-HRP. In the remaining experiments, the two labels were examined separately. Serotonin-like immunoreactive fibers were found throughout all layers of SC, but were most densely distributed within the zonal and upper superficial gray layers. Most 5-HT fibers were thin and had characteristic varicosities and terminal swellings. At the EM level, immunoreactive terminals and varicosities were found to contain small agranular vesicles and occasionally large granular vesicles (LGVs). Conventional synaptic densities were only rarely observed. Injections of WGA-HRP into SC resulted in labeling of neurons throughout the dorsal raphe nucleus and surrounding ventrolateral periaqueductal gray. Only a few cells were found in the raphe medianus and raphe pontis and none within the raphe magnus or other medullary raphe nuclei. Cells in the dorsal raphe giving rise to the SC projection varied in shape, size, and morphology and must represent more than one cell type. The morphology of these cells was indistinguishable from that of cells in the dorsal raphe which were double labeled by anti-5-HT and WGA-HRP. We conclude that the 5-HT innervation of the superior colliculus varies in density in different laminae, arises from several different cell types, and originates primarily from the dorsal raphe nucleus with minor projections from raphe medianus and raphe pontis.
Similar content being viewed by others
References
Agnati LF, Fuxe K, Hökfelt T, Benfenati F, Calza L, Johansson O, De Mey J (1982) Morphometric characterization of transmitter-identified nerve cell groups: analysis of mesencephalic 5-HT nerve cell bodies. Brain Res Bull 9: 45–51
Beitz AJ, Clements JRA, Mullett MA, Ecklund LJ (1986) Differential origin of brainstem serotoninergic projections to the midbrain periaqueductal gray and superior colliculus of the rat. J Comp Neurol 250: 498–509
Bermal AL (1968) The brain stem of the cat. A cytoarchitectonic atlas with stereotaxic coordinates. University Wisconsin Press, Madison, 175 pp
Bobillier P, Petitjean F, Salvert D, Ligier M, Seguin S (1975) Differential projections of the nucleus raphe dorsalis and nucleus raphe centralis as revealed by autoradiography. Brain Res 85: 205–210
Bobillier P, Seguin S, Petitjean F, Salvert D, Touret M, Jouvet M (1976) The raphe nuclei of the cat brain stem: a topographical atlas of their efferent projections as revealed by autoradiography. Brain Res 113: 449–486
Chan-Palay V, Jonsson G, Palay SL (1978) Serotonin and substance P coexist in neurons of the rat's central nervous system. Proc Natl Acad Sci (USA) 75: 1582–1586
De Lima AD, Singer W (1987) The serotonergic fibers in the dorsal lateral geniculate nucleus of the cat: distribution and synaptic connections demonstrated with immunocytochemistry. J Comp Neurol 258: 339–351
Diaz-Cintra S, Cintra L, Kemper T, Resnick O, Morgane PJ (1981) Nucleus raphe dorsalis: a morphometric Golgi study in rats of three age groups. Brain Res 207: 1–16
Edwards SB, Ginsburgh CL, Henkel CK, Stein BE (1979) Sources of subcortical projections to the superior colliculus in the cat. J Comp Neurol 184: 309–330
Fuxe K (1965) Evidence for the existence of monoamine neurons in the central nervous system. IV. Distribution of monoamine nerve terminals in the central nervous system. Acta Physiol Scand 64 [Suppl] 247: 38–85
Glazer EJ, Steinbusch H, Verhofstad A, Basbaum AI (1981) Serotonin neurons in nucleus raphe dorsalis and paragigantocellularis of the cat contain enkephalin. J Physiol (Paris) 77: 241–245
Hoffmann KP (1973) Conduction velocity in pathways from retina to superior colliculus in the cat: a correlation with receptivefield properties. J Neurophysiol 36: 409–424
Huerta MF, Harting JK (1984) The mammalian superior colliculus: studies of its morphology and connections. In: Vanegas H (ed) Comparative neurology of the optic tectum. Plenum, New York, pp 687–773
Jacobs BL, Heym J, Trulson ME (1981) Behavioural and physiological correlates of brain serotoninergic unit activity. J Physiol (Paris) 77: 431–436
Kawai N (1970) Release of 5-hydroxytryptamine from slices of superior colliculus by optic tract stimulation. Neuropharmacology 9: 395–397
Kawai N, Yamamoto C (1969) Effect of 5-hydroxytryptamine, LSD, and related compounds on electrical activities evoked in vitro in thin sections from the superior colliculus. Int J Neuropharmacol 8: 437–449
Leger L, Sakai K, Salvert D, Touret M, Jouvet M (1975) Delineation of dorsal lateral geniculate afferents from the cat brain as visualized by the horseradish peroxidase technique. Brain Res 93: 480–496
Maley B, Elde R (1982) Immunohistochemical localization of putative neurotransmitters within the feline nucleus tractus solitarii. Neuroscience 7: 2469–2490
Marcinkiewicz M, Verge D, Gozlan H, Pichat L, Hamon M (1984) Autoradiographic evidence for the heterogeneity of 5-HT1 sites in the rat brain. Brain Res 291: 159–163
McCarley RW, Nelson JP, Hobson JA (1978) Ponto-geniculooccipital (PGO) burst neurons: correlative evidence for neuronal generators of PGO waves. Science 201: 269–272
McCarley RW, Nelson JP, Hobson JA, Strassman A (1981) A cross-correlogram study of PGO-related neuronal activity in tegmental reticular nucleus, central tegmental field, and superior colliculus. Sleep Res 10: 38
McIlwain JT (1978) Cat superior colliculus: extracellular potentials related to W-cell synaptic actions. J Neurophysiol 41: 1343–1358
Mesulam M-M (1978) Tetramethylbenzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction-product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26: 106–117
Mize RR (1983) Variations in the retinal synapses of the cat superior colliculus revealed using quantitative electron microscope autoradiography. Brain Res 269: 211–221
Mize RR (1985a) A microcomputer plotter for use with light and electron microscopes. In: Mize RR (ed) The microcomputer in cell and neurobiology research. Elsevier, New York, pp 111–133
Mize RR (1985b) Morphometric measurement using a computerized digitizing system. In: Mize RR (ed) The microcomputer in cell and neurobiology research. Elsevier, New York, pp 177–215
Mize RR (1988) Immunocytochemical localization of gammaaminobutyric acid (GABA) in the cat superior colliculus. J Comp Neurol (in press)
Mize RR, Holdefer RN, Nabors LB (1988) Quantitative immunocytochemistry using an image analyzer. I. Hardward, image processing, and data analysis. J Neurosci Meth (in press)
Mize RR, Payne MP (1987) The innervation density of serotonergic (5-HT) fibers varies in different subdivisions of the cat lateral geniculate nucleus complex. Neurosci Lett 82: 133–139
Mize RR, Spencer RF, Sterling P (1982) Two types of GABAaccumulating neuron in the superficial gray layer of the cat superior colliculus. J Comp Neurol 206: 180–192
Molliver ME, Grzanna R, Lidov HGW, Morrison JH, Olschowka JA (1982) Monoamine systems in the cerebral cortex. In: Chan-Palay V, Palay SL (eds) Cytochemical methods in neuroanatomy. Alan R Liss, New York, pp 255–277
Moore RY, Halaris AE, Jones BE (1978) Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol 180: 417–438
Morgane PJ (1981) Monoamine theories of sleep: the role of serotonin — a review. Psychopharmacol Bull 17: 13–17
Morrison JH, Foote SL (1986) Noradrenergic and serotonergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J Comp Neurol 243: 117–138
Nanopoulos D, Belin MF, Maitre M, Vincendon G, Pujol JF (1982) Immunocytochemical evidence for the existence of GABAergic neurons in the nucleus raphe dorsalis: possible existence of neurons containing serotonin and GABA. Brain Res 232: 375–389
Nelson JP, McCarley RW, Hobson JA (1983) REM sleep burst neurons, PGO waves, and eye movement information. J Neurophysiol 50: 784–797
Okada Y, Saito M (1979) Inhibitory action of adenosine, 5-HT (serotonin) and GABA (gamma-aminobutyric acid) on the postsynaptic potential (PSP) of slices from olfactory cortex and superior colliculus in correlation to the level of cyclic AMP. Brain Res 160: 368–371
Palkovits M, Brownstein M, Saavedra JM (1974) Serotonin content of the brain stem nuclei in the rat. Brain Res 80: 237–249
Pasik P, Pasik T, Holstein GR (1988) Serotonin-immunoreactivity in the monkey lateral geniculate nucleus. Exp Brain Res 69: 662–666
Pasquier DA, Villar MJ (1982) Specific serotonergic projections to the lateral geniculate body from the lateral cell groups of the dorsal raphe nucleus. Brain Res 249: 142–146
Pickel VM, Joh TH, Chan J, Beaudet A (1984) Serotonergic terminals: ultrastructure and synaptic interaction with catecholamine-containing neurons in the medial nuclei of the solitary tracts. J Comp Neurol 225: 291–301
Ruda MA, Gobel S (1980) Ultrastructural characterization of axonal endings in the substantia gelatinosa which take up [3H] serotonin. Brain Res 184: 57–83
Saavedra JM (1977) Distribution of serotonin and synthesizing enzymes in discrete areas of the brain. Fed Proc 36: 2134–2141
Segu L, Abdelkefi J, Dusticier G, Lanoir J (1986) High-affinity serotonin binding sites: autoradiographic evidence for their location on retinal afferents in the rat superior colliculus. Brain Res 384: 205–217
Sherman SM, Spear PD (1982) Organization of visual pathways in normal and visually deprived cats. Physiol Rev 63: 738–855
Steinbusch HVM (1981) Distribution of serotonin-immunoreactivity in the central nervous system of the rat: cell bodies and terminals. Neuroscience 6: 557–618
Steinbusch HWM, Nieuwenhuys R (1983) The raphe nuclei of the rat brainstem: a cytoarchitectonic and immunohistochemical study. In: Emson PC (ed) Chemical neuroanatomy. Raven Press, New York, pp 131–207
Straschill M, Perwein J (1971) Effect of iontophoretically applied biogenic amines and of choliomimetic substances upon the activity of neurons in the superior colliculus and mesencephalic reticular formation of the cat. Pflügers Arch 324: 43–55
Taber-Pierce E, Foote WE, Hobson JA (1976) The efferent connection of the nucleus raphe dorsalis. Brain Res 107: 137–144
Ueda S, Ihara N, Sano Y (1986) The organization of serotonin fibers in the mammalian superior colliculus: an immunohistochemical study. Anat Embryol 173: 13–21
Urano A (1977) Effects of eye enucleation on the activity of monoamine oxidase and acetylcholinesterase in the superior colliculus of the rat. Cell Tiss Res 179: 331–345
Wilson JR, Hendrickson AE (1988) Serotonergic axons in the monkey's lateral geniculate nucleus. Visual Neurosci 1: 125–133
Young WS, Kuhar MJ (1980) Serotonin receptor localization in rat brain by light microscopic autoradiography. Eur J Pharmacol 62: 237–239
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mize, R.R., Horner, L.H. Origin, distribution, and morphology of serotonergic afferents to the cat superior colliculus: a light and electron microscope immunocytochemistry study. Exp Brain Res 75, 83–98 (1989). https://doi.org/10.1007/BF00248533
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00248533