Abstract
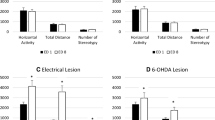
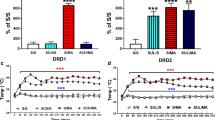
Changes taking place after unilateral 6-hydroxydopamine lesion of the dopaminergic nigrostriatal system have been studied by performing spontaneous, amphetamine-induced and apomorphine-induced rotational behaviour testing and tyrosine hydroxylase (TH) and Fos protein immunohistochemistry in the same rats. Apomorphine at a low dosage (0.25 mg/kg) induced contraversive rotation and supersensitive striatal Fos expression that were detected 24–48 h post-lesion and gradually increased in magnitude. Twenty-four hours after lesion, both high (5 mg/kg) and low doses (0.5 mg/kg) of D-amphetamine induced contraversive rotation and intense striatal Fos activation on the denervated side; however, only the higher dose induced Fos on the normal side. Two, 3 and 4 days after lesion, 0.5 mg/kg amphetamine induced contraversive rotation, but 5 mg/kg induced transitory contraversive rotation which switched to ipsiversive. In the normal striatum, only high doses of amphetamine induced Fos, but Fos induction in the denervated striatum was similar with both doses: areas showing severely decreased TH immunoreactivity still showed considerable Fos immunoreactivity, and some areas still showing TH immunoreactivity had higher Fos density than in the normal side. Seven and 14 days after lesion the loss of TH immunoreactivity and apomorphine-induced supersensitive Fos expression were more evenly distributed, and amphetamine induced only ipsiversive rotation and a low density of Fos-positive nuclei in the denervated striatum. These results indicate that the severe and progressive loss of dopaminergic terminals is counteracted by an early and rapidly progressing dopamine supersensitivity, together with a higher susceptibility to drug-induced dopamine release. This explains the apparently paradoxical contraversive rotation induced by amphetamine during the first week post-lesion. However, experiments involving successive drug injections indicated that only the first amphetamine injection releases dopamine from the lesioned terminals.
Similar content being viewed by others
References
Altar CA, Marien MR, Marshall JF (1987) Time course of adaptations in dopamine biosynthesis, metabolism, and release following nigrostriatal lesions: implications for behavioral recovery from brain injury. J Neurochem 48:390–399
Anden EE, Bedard P, Fuxe K, Ungerstedt U (1972) Early and selective increase in brain dopamine levels after axotomy. Experientia 28:300–301
Ariano MA (1988) Striatal dopamine receptor distribution following chemical lesion of the nigrostriatal pathway. Brain Res 443:204–214
Buonamici M, Caccia C, Carpentieri M, Pegrassi L, Rossi AC, Di Chiara G (1986) D1 receptor supersensitivity in the rat striatum after unilateral 6-bydroxydopamine lesions. Eur J Pharmacol 126:347–348
Carey RJ (1992) Factors in amphetamine-induced contralateral rotation in the unilateral 6-OHDA lesion rat model during the first-week postoperative: implications for neuropathology and neural grafting. Brain Res 570:11–20
Castañeda E, Whishaw IQ, Robinson TE (1990) Changes in striatal dopamine neurotransmission assessed with microdialysis following recovery from a bilateral 6-OHDA lesion: variation as a function of lesion size. J Neurosci 10:1847–1854
Cenci MA, Kalen P, Mandel RJ, Wictorin K, Björklund A (1992) Dopaminergic transplants normalize amphetamine- and apomorphine-induced Fos expression in the 6-hydroxydopamine lesioned striatum. Neuroscience 46:943–957
Creese I, Snyder AH (1979) Nigrostriatal lesions enhance striatal [3H]apomorphine and [3H]spiroperidol binding. Eur J Pharmacol 56:277–281
Creese I, Burt DR, Snyder SH (1977) Dopamine receptor binding enhancement accompanies lesion-induced behavioural supersensitivity. Science 197:596–598
Dragunow M, Leah JD, Faull RLM (1991) Prolonged and selective induction of Fos-related antigen(s) in striatal neurons after 6-hydroxydopamine lesions of the rat substantia nigra pars compacta. Mol Brain Res 10:355–358
Ewing AG, Wightman RM (1984) Monitoring the stimulated release of dopamine with in vivo voltammetry. II. Clearance of released dopamine from extracellular fluid. J Neurochem 43:570–577
Graybiel AM, Moratalla R, Robertson HA (1990) Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA 87:6912–6916
Hefti F, Melamed E, Sahakian BJ, Wurtman RJ (1980a) Circling behavior in rats with partial, unilateral nigro-striatal lesions: effect of amphetamine, apomorphine, and DOPA. Pharmacol Biochem Behav 12:185–188
Hefti F, Melamed E, Wurtman RJ (1980b) Partial lesions of the dopaminergic nigrostriatal system in rat brain: biochemical characterization. Brain Res 195:123–137
Hokfelt T, Ungerstedt U (1973) Effects of 6-hydroxydopamine on central monoamine neurons with special reference to the nigro-striatal dopamine system: an electron and fluorescence microscopical study. Brain Res 60:269–297
Labandeira-Garcia JL, Wictorin K, Cunningham ET, Björklund A (1991) Development of intrastriatal striatal grafts and their afferent innervation from the host. Neuroscience 42:407–426
Labandeira-Garcia JL, Tobio JP, Guerra MJ (1994) Comparison between normal developing striatum and developing striatal grafts using drug-induced Fos expression and neuron-specific enolase immunohistochemistry. Neuroscience 60:399–415
Labandeira-Garcia JL, Liste I, Tobio JP, Rozas G, Lopez-Martin E, Guerra MJ (1995) Intrathalamic striatal grafts survive and affect circling behaviour in adult rats with excitotoxically lesioned striatum. Neuroscience, 68:737–749
LaHoste GJ, Marshall JF (1992) Dopamine supersensitivity and D1/D2 synergism are unrelated to changes in striatal receptor density. Synapse 12:14–26
LaHoste GJ, Yu J, Marshall JF (1993) Striatal fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivity. Proc Natl Acad Sci USA 90:7451–7455
Langeloh A, Bonisch H, Trendelenburg U (1987) The mechanism of the [3H]noradrenaline releasing effects of various substrates of uptake: multifactorial induction of outward transport. Naunyn-Schmiedeberg's Arch Pharmacol 336:602–610
Liu FC, Graybiel AM, Dunnett SB, Baughman RW (1990) Intrastriatal grafts derived from fetal striatal primordia. II. Reconstitution of cholinergic and dopaminergic systems. J Comp Neurol 295:1–14
Mintz M, Douglas RJ, Tomer R, Villiers AS de, Kellaway L (1986) Transient contralateral rotation following unilateral substantia nigra lesion reflects susceptibility of the nigrostriatal system to exhaustion by amphetamine. Life Sci 39:69–76
Mishra RK, Marshall AM, Varmuza SL (1980) Supersensitivity in rat caudate nucleus: effects of 6-hydroxydopamine on the time course of dopamine receptor and cyclic AMP changes. Brain Res 200:47–57
Morelli M, Cozzolino A, Pinna A, Fenu S, Carta A, Di Chiara G (1993) L-Dopa stimulates c-fos expression in dopamine denervated striatum by combined activation of D-1 and D-2 receptors. Brain Res 623:334–336
Morgan JI, Curran T (1991) Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci 14:421–451
Neve KA, Kozlowski MR, Marshall JF (1982) Plasticity of neostriatal dopamine receptors after nigrostriatal injury: relationship to recovery of sensorimotor functions and behavioral supersensitivity. Brain Res 244:33–44
Neve KA, Altar CA, Wong CA, Marshall JF (1984) Quantitative analysis of [3H]spiroperidol binding to rat forebrain sections: plasticity of neostriatal dopamine receptors after nigrostriatal injury. Brain Res 302:9–18
Oberlander C, Eduard C, Dumont C, Boissier JB (1979) Circling behaviour induced by dopamine releasers and/or uptake inhibitors during degeneration of the nigrostriatal pathway. Eur J Pharmacol 60:163–170
Paul ML, Graybiel AM, David JC, Robertson HA (1992) D1-like and D2-like dopamine receptors synergistically activate rotation and c-fos expression in the dopamine-depleted striatum in a rat model of Parkinson's disease. J Neurosci 12:3729–3742
Paxinos G, Watson C (1985) The rat brain in stereotaxic coordinates. Academic Press, New York
Pycock CJ (1980) Turning behavior in animals. Neuroscience 5:461–514
Robertson HA, Peterson MR, Murphy K, Robertson GS (1989) D1-dopamine receptor agonists selectively activate striatal c-fos independent of rotational behaviour. Brain Res 503:346–349
Robertson GS, Vincent SR, Fibiger HC (1992) D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience 49:285–296
Robinson TE, Becker JB (1983) The rotational behavior model: asymmetry in the effects of unilateral 6-OHDA lesions of the substantia nigra in rats. Brain Res 264:127–131
Robinson TE, Mocsary Z, Camp DM, Whishaw IQ (1994a) Time course of recovery of extracellular dopamine following partial damage to the nigrostriatal dopamine system. J Neurosci14:2687–2696
Robinson TE, Noordhoorn M, Chan EM, Mocsary Z, Camp DM, Whishaw IQ (1994b) Relationship between asymmetries in striatal dopamine release and the direction of amphetamine-induced rotation during the first week following a unilateral 6OHDA lesion of the substantia nigra. Synapse 17:16–25
Savasta M, Dubois A, Feuerstein C, Benavides J, Scatton B (1987) Localization of D1 dopamine receptors in the rat brain by quantitative autoradiography: effect of dopaminergic denervation. Biogenic Amines 4:419–429
Schwarting RKW, Bonatz AE, Carey RJ, Huston JP (1991) Relationships between indices of behavioral asymmetries and neurochemical changes following mesencephalic 6-hydroxydopamine injections. Brain Res 554:46–55
Staunton DA, Wolfe BB, Groves PM, Molinoff PB (1981) Dopamine receptor changes following destruction of the nigrostriatal pathway: lack of a relationship to rotational behavior. Brain Res 211:315–327
Trugman JM, James CL (1992) Rapid development of dopaminergic supersensitivity in reserpine-treated rats demonstrated with 14C-2-deoxyglucose autoradiography. J Neurosci 12:2875–2879
Ungerstedt U (1971a) Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl 367:69–93
Ungerstedt U (1971b) Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behaviour. Acta Physiol Scand Suppl 367:49–68
Ungerstedt U, Arbuthnott GW (1970) Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res 24:485–493
Vaccarino FJ, Franklin KBJ, Prupas D (1985) Opposite locomotor asymmetries elicited from medial and lateral substantia nigra: role of the superior colliculus. Physiol Behav 35:741–747
Waddington JL, Cross AJ, Longden A, Owen F, Poulter M (1979) Apomorphine-induced rotation in the unilateral 6-OHDA-lesioned rat: relationship to changes in striatal adenylate cyclase activity and 2H-spiperone binding. Neuropharmacology 18:643–645
Weiser M, Baker H, Wessel TC, Joh TH (1993) Differential spatial and temporal gene expression in response to axotomy and de-afferentation following transection of the medial forebrain bundle. J Neurosci 13:3472–3484
Winkler JD, Weiss B (1989) Effect of continuous exposure to selective D1 and D2 dopaminergic agonists on rotational behavior in supersensitive mice. J Pharmacol Exp Ther 249:507–516
Zhang WQ, Tilson HA, Nanry KP, Hudson PM, Hong JS, Stachowiak MK (1988) Increased dopamine release from striata of rats after unilateral nigrostriatal bundle damage. Brain Res 461:335–342
Zigmond MJ, Acheson AL, Stachowiak MK, Strickerm EM (1984) Neurochemical compensation after nigrostriatal bundle injury in an animal model of preclinical parkinsonism. Arch Neurol 41:856–861
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Labandeira-Garcia, J.L., Rozas, G., Lopez-Martin, E. et al. Time course of striatal changes induced by 6-hydroxydopamine lesion of the nigrostriatal pathway, as studied by combined evaluation of rotational behaviour and striatal Fos expression. Exp Brain Res 108, 69–84 (1996). https://doi.org/10.1007/BF00242905
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00242905