Abstract
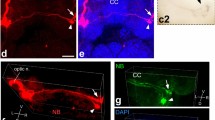
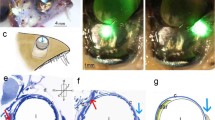
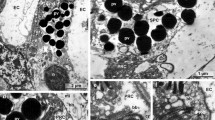
According to Sperry's chemoaffinity hypothesis, the projection of a small eye fragment with a reduced amount of optic fibres should be restricted to that position in the optic tectum corresponding to its own specificity. However, previous investigations on different types of quarter-eyes in Xenopus laevis have revealed that their retinal projection was always restricted to the rostral part of the tectum, no matter what the origin of the remaining retinal quadrant. To get an indication of the state of specificity in such eye fragments, we investigated by electrophysiological and histological methods several features of the retinal projections of temporoventral (TV), naso-ventral (NV) and ventral (V) quarter-eyes which referred to their positional identity. Irrespective of their different origins, the projections were always located in the rostral part of the tectum, the size of the innervated tectal area depending for all fragment types on the size of the quarter-eyes, i.e. number of optic fibres. However, quantitative analyses revealed that with increasing eye size the various fragments expand their projections preferentially into those tectal areas that match their original specificity: TV projection is more concentrated in the rostral tectum, NV eyes expand their projections mainly to the caudal tectum, and V eyes enlarge their projections equally into the medial and caudal tectum. In addition, fibre-tracing experiments with cobaltic lysine showed that, according to the different origins of the quarter-eyes, retinal fibres follow the appropriate branch of the optic tract selectively: fibres of NV and V eyes pass mainly through the medial tract, and most fibres of TV eyes innervate the rostral tectum directly from a central position between the two side branches. All these findings suggest that the different types of quarter-eyes retain their original positional identity. Thus, their rostrally located retinotectal projections are not in register with their retinal specificity. We conclude that in X. laevis local positional markers in the tectum, if present at all, do not influence the development of the retinotectal projection. Instead we suggest a concept of self-sorting of the optic fibres, which can account for the partial innervation of the rostral tectum in different types of quarter-eyes.
Similar content being viewed by others
References
Baier H, Bonhoeffer F (1992) Axon guidance by gradients of a target-derived component. Science 255:472–475
Berman N, Hunt RK (1975) Visual projections to the optic tecta in Xenopus after partial extirpation of the embryonic eye. J Comp Neurol 162:23–42
Chung SH, Cooke J (1975) Polarity of structure and of ordered nerve connections in the developing amphibian brain. Nature 258:126–132
Cline HT, Constantine-Paton M (1990) NMDA receptor agonist and antagonists alter retinal ganglion cell arbor structure in the developing frog retinotectal projection. J Neurosci 10:1197–1216
Cook JE, Horder TJ (1977) The multiple factors determining retinotopic order in the growth of optic fibres into the optic tectum. Phil Trans R Soc Lond [Biol] 278:261–276
Cooke J, Gaze RM (1983) The positional coding system in the early eye rudiment of Xenopus laevis, and its modification after grafting operations. J Embryol Exp Morphol 77:53–71
Degen N, Brändle K (1986) The retinotectal projection of quarter eyes in Xenopus laevis. Dev Brain Res 29:141–143
Degen N, Brändle K, Peter L (1993) Histological studies on the development of retinotectal projections from nasoventral quarter-eyes in Xenopus laevis. Development 118:589–599
Fawcett JW, Gaze RM (1982) The retinotectal fibre pathways from normal and compound eyes in Xenopus. J Embryol Exp Morphol 72:19–37
Feldman JD, Gaze RM (1975) The development of half-eyes in Xenopus tadpoles. J Comp Neurol 162:13–22
Fraser SE, Perkel DH (1990) Competitive and positional cues in the patterning of nerve connections. J Neurobiol 21:51–72
Fujisawa H (1987) Mode of growth of retinal axons within the tectum of Xenopus tadpoles, and implications in the ordered neuronal connection between the retina and the tectum. J Comp Neurol 260:127–139
Gaze RM, Keating MJ, Chung SH (1974) The evolution of the retinotectal map during development in Xenopus. Proc R Soc Lond [Biol] 185:301–330
Gaze RM, Feldman JD, Cooke J, Chung SH (1979) The orientation of the visuotectal map in Xenopus: developmental aspects. J Embryol Exp Morphol 53:39–66
Goodman CS, Shatz CJ (1993) Developmental mechanisms that generate precise patterns of neuronal connectivity. Neuron [Suppl] 10:77–98
Görcs T, Antal M, Olan E, Székely G (1979) An improved cobalt labelling technique with complex compounds. Acta Biol Hung 30:79–86
Häse J, Degen N, Brändle K (1991) Correlation between cell proliferation in eye fragments and duplicated retinotectal projections: indication for an epimorphic regulation of positional markers in the retina of Xenopus laevis. Soc Neurosci Abstr 17(2): 1133
Holt CE (1980) Cell movements in Xenopus eye development. Nature 287:850–852
Holt CE, Harris WA (1983) Order in the initial retinotectal map in Xenopus: a new technique for labelling growing nerve fibers. Nature 301:150–152
Holt CE (1984) Does timing of axon outgrowth influence initial retinotectal topography in Xenopus? J Neurosci 4:1130–1152
Holt CE, Bertsch TW, Ellis HM, Harris WA (1988) Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron 1:15–26
Horder TJ, Martin KAC (1978) Morphogenetics as an alternative to chemospecificity in the formation of nerve connections. In: Curtis ASG (ed) Cell-cell recognition. Soc Exp Biol Symp, Cambridge University Press, Cambridge, pp 275–358
Ide CF, Kosofsky E, Hunt RK (1979) Control of pattern duplication in the retinotectal system of Xenopus. Dev Biol 69:337–360
Ide CF, Reynolds P, Tompkins R (1984) Two healing patterns correlate with different adult neural connectivity patterns in regenerating embryonic Xenopus retina. J Exp Zool 230:71–80
Jacobson M (1967) Retinal ganglion cells: specification of central connections in larval Xenopus laevis. Science 155:1106–1108
Langsdorf G, Brändle K (1991) A model of the growth of normal and fragmented eyes of Xenopus laevis before metamorphosis (abstract). In: Elsner N, Penzlin H (eds) Synapse-transmission-modulation. Proceedings of 19th Göttingen Neurobiology Conference. Thieme, Stuttgart, p 246
Merrill EG, Ainsworth A (1972) Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng 10:662–672
Nieuwkoop PD, Faber J (1956) A normal table of Xenopus laevis (Daudin). North-Holland, Amsterdam
Riedwyl H (1980) Regressionsgerade und Verwandtes. UTB 923. Haupt, Stuttgart
Rugh R (1962) Experimental embryology. Burgess, Minneapolis
Sachs L (1971) Statistische Auswertungsmethoden. Springer, Berlin Heidelberg New York
Schmidt JT (1978) Retinal fibers alter tectal positional markers during the expansion of the half retinal projection in goldfish. J Comp Neurol 177:279–300
Sperry RW (1963) Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci USA 50:703–710
Steedman JG, Stirling RV, Gaze RM (1979) The central pathways of optic fibres in Xenopus tadpoles. J Embryol Exp Morphol 50:199–215
Straznicky C, Gaze RM, Keating MJ (1980) The retinotectal projections from surgically rounded-up half-eyes in Xenopus. J Embryol Exp Morphol 58:79–91
Straznicky C, Gaze RM, Keating MJ (1981) The development of the retinotectal projections from compound eyes in Xenopus. J Embryol Exp Morphol 62:13–35
Straznicky C, Gaze RM (1982) The innervation of a virgin tectum by a double-temporal or a double-nasal eye in Xenopus. J Embryol Exp Morphol 68:9–21
Székely G, Lázár G (1976) Cellular and synaptic architecture of the optic tectum. In: Llinas R, Precht W (eds) Frog neurobiology. Springer, Berlin Heidelberg New York, pp 407–435
Willshaw DJ, Malsburg C van der (1979) A marker induction mechanism for the establishment of ordered neural mappings: its application to the retinotectal problem. Phil Trans R Soc Lond [Biol] 287:203–243
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brändle, K., Degen, N. Retinal specificity in eye fragments: investigations on the retinotectal projections of different quarter-eyes in Xenopus laevis . Exp Brain Res 102, 272–286 (1994). https://doi.org/10.1007/BF00227514
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227514