Summary
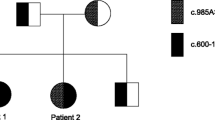
A series of experiments has established the molecular defect in the medium-chain acyl-coenzyme A (CoA) dehydrogenase (MCAD) gene in a family with MCAD deficiency. Demonstration of intra-mitochondrial mature MCAD indistinguishable in size (42.5-kDa) from control MCAD, and of mRNA with the correct size of 2.4 kb, indicated a point-mutation in the coding region of the MCAD gene to be disease-causing. Consequently, cloning and DNA sequencing of polymerase chain reaction (PCR) amplified complementary DNA (cDNA) from messenger RNA of fibroblasts from the patient and family members were performed. All clones sequenced from the patient exhibited a single base substitution from adenine (A) to guanine (G) at position 985 in the MCAD cDNA as the only consistent base-variation compared with control cDNA. In contrast, the parents contained cDNA with the normal and the mutated sequence, revealing their obligate carrier status. Allelic homozygosity in the patient and heterozygosity for the mutation in the parents were established by a modified PCR reaction, introducing a cleavage site for the restriction endonuclease NcoI into amplified genomic DNA containing G985. The same assay consistently revealed A985 in genomic DNA from 26 control individuals. The A to G mutation was introduced into an E. coli expression vector producing mutant MCAD, which was demonstrated to be inactive, probably because of the inability to form active tetrameric MCAD. All the experiments are consistent with the contention that the G985 mutation, resulting in a lysine to glutamate shift at position 329 in the MCAD polypeptide chain, is the genetic cause of MCAD deficiency in this family. We found the same mutation in homozygous form in 11 out of 12 other patients with verified MCAD deficiency.
Similar content being viewed by others
References
Akeson AL, Wiginton DA, States JC, Perme CM, Dusing MR, Hutton JJ (1987) Mutations in the human adenosine deaminase gene that affect protein structure and RNA splicing. Proc Natl Acad Sci (USA) 84:5947–5951
Bennett MJ, Allison F, Pollitt RJ, Variend S (1990) Fatty acid oxidation defects as causes of unexpected death in infancy. Im: Tanaka K, Coates PM (eds) Fatty acid oxidation: clinical, biochemical and molecular aspects. Liss, New York, p 349
Bross P, Engst J, Strauss AW, Kelly DP, Rasched I, Ghisla S (1990) Characterization of wild-type and an active site mutant of human medium-chain acyl-CoA dehydrogenase after expression in E. coli. J Biol Chem 265:7116–7119
Burnette WN (1981) Western blotting: electrophoretic transfer of proteins from SDS-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 112:195–203
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Fourney RM, Miyakoshi J, Day III RS, Paterson MC (1988) Northern blotting: efficient RNA staining and transfer. Focus (Gibco BRL) 10:5–7
Gregersen N (1985) The acyl-CoA dehydrogenation deficiencies. Scand J Clin Lab Invest 45 [Suppl 174]: 1–60
Gregersen N, Lauritzen R, Rasmussen K (1976) Suberylglycine excretion in the urine of a patient with dicarboxylic aciduria. Clin Chim Acta 70:417–425
Gregersen N, Rosleff F, Kølvraa S, Hobolth N, Rasmussen K, Lauritzen R (1980) Non-ketotic C6-C10-dicarboxylic aciduria: biochemical investigation of two cases. Clin Chem Acta 102: 179–189
Gregersen N, Kølvraa S, Rasmussen K, Mortensen PB, Divry P, David M, Hobolth N (1983) General (medium-chain) acyl-CoA dehydrogenase deficiency (non-ketotic dicarboxylic aciduria): quantitative urinary excretion pattern of 23 biologically significant organic acids in three cases. Clin Chim Acta 132:181–191
Gregersen N, Koch J, Kølvraa S, Petersen KB, Bolund L (1987) Improved methods for the detection of unique sequences in Southern blots of mammalian DNA by non-radioactive biotinylated DNA hybridization probes. Clin Chim Acta 169:267–280
Ikeda Y, Tanaka K (1990a) Purification and characterization of five acyl-CoA dehydrogenases from rat liver mitochondria. In: Tanaka K, Coates PM (eds) Fatty acid oxidation: clinical, biochemical and molecular aspects. Liss, New York, pp 37–54
Ikeda Y, Tanaka K (1990b) In vitro translation, mitochondrial uptake and post-translational processing of four acyl-CoA dehydrogenases. In: Tanaka K, Coates P (eds) Fatty acid oxidation: clinical, biochemical and molecular aspects. Liss, New York, pp 55–68
Ikeda Y, Hale DE, Keese SM, Coates PM, Tanaka K (1986) Biosynthesis of variant medium-chain acyl-CoA dehydrogenase in cultured fibroblasts from patients with medium-chain acyl-CoA dehydrogenase deficiency. Pediatr Res 20:843–847
Kelly DP, Kim JJ, Billadello JJ, Hainline BE, Chu TW, Strauss AW (1987) Nucleotide sequence of medium-chain acyl-CoA dehydrogenase mRNA and its expression in enzyme-deficient human tissue. Proc Natl Acad Sci USA 84:4068–4072
Kim JJP, Wu J (1988) Structure of the medium-chain acyl-CoA dehydrogenase from pig liver mitochondria at 3-Å resolution. Proc Natl Acad Sci USA 85:6677–6681
Kølvraa S, Gregersen N, Christensen E, Hobolth N (1982) In vitro fibroblast studies in a patient with C6-C10-dicarboxylic aciduria: evidence for a defect in general acyl-CoA dehydrogenase. Clin Chim Acta 126:53–67
Manning NJ, Olpin SE, Pollitt RJ, Webley J (1990) A comparison of [9, 10-3H]palmitic and [9, 10-3H]myristic acids for the detection of defects of fatty acid oxidation in intact cultured fibroblasts. J Inherited Metab Dis 13:58–68
Matsubara Y, Kraus JP, Yang-Feng TL, Francke U, Rosenberg LE, Tanaka K (1986) Molecular cloning of cDNA encoding rat and human medium-chain acyl-CoA dehydrogenase and assignment of the gene to human chromosome 1. Proc Natl Acad Sci USA 83:6543–6547
Ohno K, Suzuki K (1988) Multiple abnormal β-hexosaminidase α-chain mRNAs in a compound heterozygous Ashkanazi Jewish patient with Tay-Sachs disease. J Biol Chem 263:18563–18567
Powell PJ, Thorpe C (1988) 2-Octynoyl coenzyme A is a mechanism-based inhibitor of pig kidney medium-chain acyl-coenzyme A dehydrogenase: isolation of the target peptide. Biochemistry 27:8022–8028
Promega (1989/1990) Protocols and application guide. Promega Co, Madison, Wis
Reiss J, Krawczak M, Schloesser M, Wagner M, Cooper DN (1990) The effect of replication errors on the mismatch analysis of PCR-amplified DNA. Nucleic Acids Res 18:973–978
Rhead WJ, Amendt BA, Fritchman KS, Felts SJ (1983) Dicarboxylic aciduria: deficient[1-14C]-octanoate oxidation and medium-chain acyl-CoA dehydrogenase activity in fibroblasts. Science 22:73–75
Rinaldo P, O'Shea JJ, Coates PM, Hale DE, Stanley CA, Tanaka K (1988) Medium-chain acyl-CoA dehydrogenase deficiency: diagnosis by stable-isotope dilution measurement of urinary n-hexanoylglycine and 3-phenylpropionylglycine. N Engl J Med 319:1308–1313
Roe CR, Coates PM (1989) Acyl-CoA dehydrogenase deficiencies. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic basis of inherited disease. McGraw-Hill, New York, p 889–914
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Schultz H (1990) Mitochondrial β-oxidation. In: Tanaka K, Coates PM (eds) Fatty acid oxidation: clinical, biochemical and molecular aspects. Liss, New York, pp 23–36
Stanley CA, Hale DE, Coates PM, Hall CL, Corkey BE, Yang W, Kelley RI, Gonzales EL, Williamson JR, Baker L (1983) Medium-chain acyl-CoA dehydrogenase deficiency in children with non-ketotic hypoglycemia and low carnitine levels. Pediatr Res 17:877–884
Strauss AW, Duran M, Zhang Z, Alpers R, Kelly D (1990) Molecular analysis of medium chain acyl-CoA dehydrogenase deficiency. In: Tanaka K, Coates PM (eds) Fatty acid oxidation: clinical, biochemical and molecular aspects. Liss, New York, p 609–623
Tindall KR, Kunkel TA (1988) Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry 27:6008–6013
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gregersen, N., Andresen, B.S., Bross, P. et al. Molecular characterization of medium-chain acyl-CoA dehydrogenase (MCAD) deficiency: identification of a lys329 to glu mutation in the MCAD gene, and expression of inactive mutant enzyme protein in E. coli . Hum Genet 86, 545–551 (1991). https://doi.org/10.1007/BF00201539
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00201539