Abstract
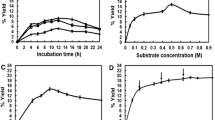
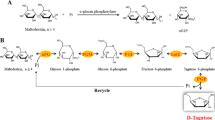
During a screening for new microbial α-glucan phosphorylases corynebacteria were found to be promising, not-yet-identified producers of these particular enzymes. A maltodextrin phosphorylase (MDP) from Corynebacterium callunae was isolated, partially characterized, and used for the production of glucose-1-phosphate (G-1-P) from different α-glucans. In fermentor cultivations of C. callunae using maltodextrin as the inducing carbohydrate component, an MDP activity of approximately 8–10 units/g biomass (equivalent to 250 units/l) could be obtained. Contaminating activities of phosphoglucomutase and phosphatase were removed by ammonium sulphate precipitation followed by hydrophobic interaction chromatography on phenyl-sepharose. The partially (14-fold) purified MDP showed pH optima of 6.8 and 6.0 in the direction of phosphorolysis and synthesis, respectively. In the presence of 50mm inorganic phosphate the enzyme was stable for more than 2 months at room temperature. The new MDP is capable of producing G-1-P from maltodextrins, soluble starch, and glycogen with decreasing order of activity. The same glucans were accepted as primers in the direction of synthesis. Increasing pH values favoured the formation of G-1-P and optimized conditions for its production were established at a pH of 7.5. The maximum attainable yields of G-1-P by the action of MDP are limited by mainly two factors: (1) no more than approximately 20% of the initial inorganic phosphate could be converted into G-1-P and (2) the highest degrees of phosphorolytic maltodextrin degradation were in the range 30–35%. These values could be increased to more than 60% after pretreatment of the maltodextrins with pullulanase.
Similar content being viewed by others
References
Arden SB, Barksdale L (1974) Glucose-1-phosphate induced accumulation of intracellular starch: a distinguishing feature of certain Corynebacteria. Int J Syst Bacteriol 24:139–141
Bergmeyer HU, Grassl M, Walter HE (1988) Phosphorylase a. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 3rd edn, vol 2. VCH, Weinheim, pp 293–295
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72:248–254
Bretaudiere JP, Spillman Th (1988) Alkaline phosphatases. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 3rd edn, vol 4. VCH, Weinheim, pp 75–92
Carrier BE, McClesky CS (1961) Intracellular starch formation in Corynebacteria. J. Bacteriol 83:1029–1036
Chen GS, Segel IH (1968) Purification and properties of glycogen phosphorylase from Escherichia coli. Arch Biochem Biophys 127:175–186
Choi Y-L, Kawamukai M, Utsumi R, Sakai H, Komano T (1989) Molecular cloning and sequencing of the glycogen phosphorylase gene from Escherichia coli. FEBS Lett 243:193–198
Guibert A, Monsan P (1988) Production and purification of sucrose phosphorylase from Leuconostoc mesenteroides. Ann NY Acad Sci 542:307–311
Hollo J, Laszlo E, Hoschke A (1971) Plant α-1,4-glucan-phosphorylases. In: Bognar R, Bruckner V, Szantay Cs (eds) Recent developments in the chemistry of natural carbon compounds, vol III. Springer, Berlin Heidelberg New York
Kamogashira T, Sugawara M, Takano M (1988) Isolation of a hypersensitive mutant and production of α-d-glucose-1-phosphate from Bacillus sp. BA-3796 screened by its use. J Ferment Technol 66:649–655
Kayane S, Kawai T, Sakata M, Imamura T, Tanigaki M, Kurosaki T (1989) Process for preparing glucose-1-phosphate. EP 0 305 981 A2; Int. Cl. C12P 19/02, C12N 11/08
Kitamoto Y, Ahashi H, Tanaka H, Mori N (1988) α-Glucose-1-phosphate formation by a novel trehalose phosphorylase from Flammulina velutipes. FEMS Microbiol Lett 552:147–149
Lindner D, Kurz G, Wallenfels K (1976) α-1,4-Glucan phosphorylase from Klebsiella pneumoniae. Purification, subunit structure and amino acid composition. Eur J Biochem 70:291–303
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Biochem 31:426–428
Norrman J, Wöber G (1974) Comparative biochemistry of α-glucan utilization in Pseudomonas amylodermosa and Pseudomonas saccharophila. Arch Microbiol 102:253–260
Palm D, Goerl R, Burger KL (1985) Evolution of catalytic and regulatory sites in phosphorylases. Nature 313:500–502
Palm D, Goerl R, Weidinger G, Zeier R, Fischer B, Schinzel R (1987) E. coli maltodextrin phosphorylase: primary structure and deletion mapping of the C-terminal site. Z Naturforsch 42c:394–400
Parish C, Cowden WB, Willenborg DO (1990) Phosphosugar-based anti-inflammatory and/or immunosuppressive drugs. WO 90/01938; Int. Cl. A61K 31/725, 31/71, CO7H 11/04
Schächtele KH, Schiltz E, Palm D (1978) Amino-acid sequence of the pyridoxal-phosphate-binding site in Escherichia coli malto dextrin phosphorylase. Eur J Biochem 92:427–435
Schinzel R, Palm D, Schnackerz KD (1992) Pyridoxal 5′-phosphate as a 31P reporter observing functional changes in the active site of Escherichia coli maltodextrin phosphorylase after site-directed mutagenesis. Biochemistry 31:4128–4133
Schwartz M, Hofnung M (1967) Maltodextrin phosphorylase from Escherichia coli. Eur J Biochem 2:132–145
VanDam HE, Duijverman P, Kieboom APG, VanBekkum H (1987) Oxidation of glucose-1-phosphate into glucuronic acid-1-phosphate using diffusion stabilized catalysts. Appl Catalysis 33:373–382
Vandamme E, Loo J van, Machtelinckx L, Laporte A de (1987) Microbial sucrose phosphorylase: fermentation process properties, and biotechnical applications. Adv Appl Microbiol 32:163–201
Wood JB, Rainbow C (1961) The maltophosphorylase of beer Lactobacilli. Biochem J 78:204–209
Yu F, Jen Y, Takeuchi E, Inouye M, Nakayama H, Tagaya M, Fukui T (1988) α-Glucan phosphorylase from Escherichia coli. J Biol Chem 263:13706–13711
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weinhäusel, A., Nidetzky, B., Rohrbach, M. et al. A new maltodextrin-phosphorylase from Corynebacterium callunae for the production of glucose-1-phosphate. Appl Microbiol Biotechnol 41, 510–516 (1994). https://doi.org/10.1007/BF00178481
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00178481