Summary
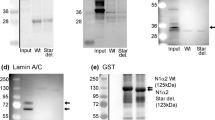
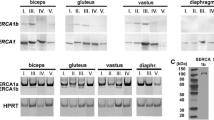
The location of porin-type1 proteins in mammalian striated muscle has been assessed using immunogold electron microscopy with an anti-porin 31HL monoclonal antibody as the primary antibody. Gold particles were found on the mitochondrial outer membrane, the sarcoplasmic reticulum and plasmalemma in longitudinal sections of rat and rabbit skeletal muscle and rabbit and sheep cardiac muscle. The relative densities of gold particles in the mitochondrial outer membrane, sarcoplasmic reticulum and plasmalemma were 7:3:1 in white sternomastoid muscle, for example. Skeletal and cardiac sarcoplasmic reticulum vesicles, which had been fractionated by discontinuous sucrose density centrifugation, were subjected to SDS-polyacrylamide gel electrophoresis and Western blotting. The anti-porin 31HL monoclonal antibody detected a band of relative molecular mass (Mr) 31 000 in all muscle sarcoplasmic reticulum vesicle fractions and also in liver mitochondria. The intensity of immunostaining of the sarcoplasmic reticulum fractions was 2.5–10% that of mitochondrial outer membranes per μg of membrane protein blotted. Contamination of the sacroplasmic reticulum fractions by mitochondrial outer membrane was <0.75% as determined from the specific activity of monoamine oxidase. Thus, only a small part of the porin detected in sarcoplasmic reticulum vesicles can be attributed to mitochondrial contamination. These results show that porin-type1 immunoreactivity is not restricted to mitochondria but found in the sarcoplasmic reticulum and plasmalemma of both mammalian skeletal and cardiac muscle.
Similar content being viewed by others
References
ADRIAN, R. H. & BRYANT, S. H. (1974) On the repetitive discharge in myotonic muscle fibres. J. Physiol. 240, 505–15.
BABEL, D., WALTER, G., GOTZ, H., THINNES, F. P., JURGENS, L., KONIG, U. & HILSCHMANN, N. (1991) Studies on human porin. VI. Production and characterization of eight monoclonal mouse antibodies against the human VDAC ‘Porin 31HL’ and their application for histotopological studies in human skeletal muscle. Biol. Chem. Hoppe Seyler 372, 1027–34.
BENSADOUN, A. & WEINSTEIN, D. (1976) Assay of proteins in the presence of interfering materials. Anal. Biochem. 70, 241–50.
BENZ, R., MAIER, E., THINNES, F. P., GOTZ, H. & HILSCHMANN, N. (1992) Studies on human porin. VII. The channel properties of the human B-lymphocyte membrane-derived ‘Porin 31HL’ are similar to those of mitochondrial porins. Biol. Chem. Hoppe Seyler 373, 295–303.
BLACHLY-DYSON, E., ZAMBRONICZ, E. B., YU, W. H., ADAMS, V., MCCABE, E. R. B., ADELMAN, J., COLOMBINI, M. & FORTE, M. (1993) Cloning and functional expression in yeast of two human isoforms of the outer mitochondrial membrane channel, the voltagedependent anion channel. J. Biol. Chem. 268, 1835–41.
BLACHLY-DYSON, E., BALDINI, A., LITT, M., MCCABE, E. R. B. & FORTE, M. (1994) Human genes encoding the voltage-dependent anion channel (VDAC) of the outer mitochondrial membrane: mapping and identification of two new isoforms. Genomics 20, 62–7.
BOSMA, M. A. (1989) Anion channels with multiple conductance levels in a mouse B lymphocyte cell line. J. Physiol 410, 67–90.
BRDICZKA, D., DOLKEN, G., KREBS, W. & HOFMANN, D. (1974) The inner boundary membrane of mitochondria. Localization and biochemical characterization, possible functions in biogenesis and metabolism. Hoppe-Seyler's Z. Physiol. Chem. 355, 731–43.
BUREAU, M. H., KHRESTCHATISKY, M., HEEREN, M. A., ZAMBROWICZ, E. B., KIM, H., GRISAR, T. M., COLOMBINI, M., TOBIN, A. J. & OLSEN, R. W. (1992) Isolation and cloning of a voltage-dependent anion channel-like Mr 36 000 polypeptide from mammlian brain. J. Biol. Chem. 267 8679–84.
CHAMBERLAIN, B. K. & FLEISCHER, S. (1988) Isolation of canine cardiac sarcoplasmic reticulum. Methods Enzymol. 157, 91–9.
COLE, T., AWNI, L. A., NYAKATURA, E., GOTZ, H., WALTER, G., THINNES, F. P. & HILSCHMANN, N. (1992) Studies on human porin. VIII. Expression of ‘Porin 31HL’ channels in the plasmalemma of the acutelymphoblastic-leukemia cell line KM3 as revealed by light- and electron-microscopy. Biol. Chem. Hoppe Seyler 373, 891–6.
COLOMBINI, M. (1994) Anion channels in the mitochondrial outer membrane. Curr. Top. Membr. 42, 73–101.
COMTE, J. & GAUTHERON, D. C. (1979) Preparation of outer membrane from pig heart mitochondria. Methods Enzymol. 55, 98–104.
COULOMBE, A., DUCLOHIER, H., CORABOEUF, E. & TOUZET, N. (1987) Single chloride-permeable channels of large conductance in cultured cardiac cells of new-born rats. Eur. Biophys. J. 14, 155–62.
De, PINTO, V., LUDWIG, O., KRAUSE, J., BENZ, R. & PALMIERI, F. (1987) Porin pores of mitochondrial outer membranes from high and low eukaryotic cells: biochemical and biophysical characterization. Biochim. Biophys. Acta 894, 109–19.
DERMIETZEL, R., HWANG, T. K., BUETTNER, R., HOFER, A., DOTZLER, E., KREMER, M., DEUTZMANN, R., THINNES, F. P., FISHMAN, G. I., SPRAY, D. C. & SIEMEN, D. (1994) Cloning and in situ localization of a brainderived porin that constitutes a large-conductance anion channel in astrocytic plasma membranes. Proc. Natl. Acad. Sci. USA 91, 499–503.
DULHUNTY, A. F. (1978) The dependence of membrane potential on extracellalar chloride concentration in mammalian skeletal muscle fibres. J. Physiol. 276, 67–82.
DULHUNTY, A. F., BANYARD, M. R. C. & MEDVECZKY, C. J. (1987) Distribution of calcium ATPase in the sarcoplasmic reticulum of fast- and slow-twitch muscles determined with monoclonal antibodies. J. Membrane Biol. 99, 79–92.
DULHUNTY, A. F., JUNANKAR, P. R. & STANHOPE, C. (1993) Immunogold labelling of calcium ATPase in the sarcoplasmic reticulum of skeletal muscle: use of 1 nm, 5 nm and 10 nm gold. J. Histochem. Cytochem. 41, 1459–66.
EISENBERG, B. R. (1982) Quantitative ultrastructure of mammalian skeletal muscle. In Handbook of physiology-skeletal muscle Section 10 (edited by PEACHEY, L. D. & ADRIAN, R.) pp. 73–112. Bethesda, MD: American Physiological Society.
GREENAWALT, J. W. (1979) Survey and update of outer and inner mitochondrial membrane separation. Methods Enzymol. 55, 88–98.
GREENAWALT, J. W. & SCHNAITMAN, C. (1970) An appraisal of the use of monoamine oxidase as an enzyme marker for the outer membrane of the rat liver mitochondria. J. Cell Biol. 46, 173–9.
HA, H., HAJEK, P., BEDWELL, D. M. & BURROWS, P. D. (1993) A mitochondrial porin cDNA predicts the existence of multiple human porins. J. Biol. Chem. 268, 12143–9.
HALS, G. D., STEIN, P. G. & PALADE, P. T. (1989) Single channel characteristics of a high conductance anion channel in ‘sarcoballs’. J. Gen. Physiol. 93, 385–410.
HAYAT, M. A. (1989) Principles and Techniques of Electron Microscopy. Biological Applications, 3rd ed. London: Macmillan.
HEIDEN, M., THINNES, F. P., KRICK, W. & HILSCHMANN, N. (1993) Influence of cytosol from various tissues on the behaviour of human porin in lipid bilayer experiments. Biol. Chem. Hoppe Seyler 374, 149.
HOVIUS, R., LAMBRECHTS, H., NICOLAY, K. & De, KRUIJFF, B. (1990) Improved methods to isolated and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim. Biophys. Acta 1021, 217–26.
INUI, M., WANG, S., SAITO, A. & FLEISCHER, S. (1988) Junctional and longitudinal sarcoplasmic reticulum of heart muscle. Methods Enzymol. 157, 100–6.
JUNANKAR, P. R., CURTIS, S. M., PACE, S. M. & DULHUNTY, A. F. (1993) Immunogold localisation of the VDAC channel in mammalian skeletal and cardiac muscle. Proc. Aust. Physiol. Pharmacol. Soc. 24(2), 179P.
JURGENS, L., ILSEMANN, P., KRATZIN, H. D., HESSE, D., ECKART, K., THINNES, F. P. & HILSCHMANN, N. (1991) Studies on human porin. IV. The primary structurs of ‘Porin 31HM’ purified from human skeletal muscle membranes and of ‘Porin 31HL’ derived from human B lymphocyte membranes are identical. Bio. Chem. Hoppe Seyler 372, 455–63.
KAYSER, H., KRATZIN, H. D., THINNES, F. P., GOTZ, H., SCHMIDT, W. E., ECKART, K. & HILSCHMANN, N. (1989) Studies on human porin. II. Characterization and primary structure of a 31-kDa porin from human B lymphocytes (Porin 31HL). Biol. Chem. Hoppe Seyler 370, 1265–78.
KELLENBERGER, E. & HAYAT, M. A. (1991) Some basic concepts for the choice of methods. In Colloidal gold: principles, methods and applications. Vol. 3 (edited by HAYAT, M. A.) pp. 1–30. San Diego: Academic Press.
LAEMMLI, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–5.
LEWIS, T. M. & BRETAG, A. H. (1991) A large conductance anion channel in a sarcoplasmic reticulum preparation from the semitendenosus muscle of the cane toad. Proc. Aust. Physiol. Pharmacol. Soc. 22(1), 19P.
LEWIS, T. M., DULHUNTY, A. F., JUNANKAR, P. R. & STANHOPE, C. (1992) Ultrastructure of sarcoballs on the surface of skinned amphibian skeletal muscle fibres. J. Muscle Res. Cell Motil. 13, 640–53.
LEWIS, T. M., ROBERTS, M. L. & BRETAG, A. H. (1994) Immunolabelling for VDAC, the mitochondrial voltage-dependent anion channel, on sarcoplasmic reticulum from amphibian skeletal muscle. Neurosci. Lett. 181, 83–6.
LIU, M. & COLOMBINI, M. (1991) Voltage gating of the mitochondrial outer membrane channel VDAC is regulated by a very conserved protein. Am. J. Physiol. 260, C371–4.
LIU, M. Y., TORGRIMSON, A. & COLOMBINI, M. (1994) Characterization and partial purification of the VDAC-channel-modulating protein from calf liver mitochondria. Biochim. Biophys. Acta 1185, 203–2.
MANNELLA, C. A. (1992) The ‘ins’ and ‘outs’ of mitochondrial membrane channels. Trends Biochem. Sci. 17, 315–20.
MCENERY, M. W. (1992) The mitochondrial benzodiazepine receptor: evidence for association with the voltage-dependent anion channel (VDAC). J. Bioenerg. Biomembr. 24, 63–9.
MEISSNER, G. (1983) Monovalent ion and calcium ion fluxes in sarcoplasmic reticulum. Mol. Cell. Biochem. 55, 65–82.
MEISSNER, G. & MCKINLEY, D. (1976) Permeability of sarcoplasmic reticulum membrane. The effect of changed ionic environments of Ca2+ release. J. Membrane Biol. 30, 79–98.
MORTON, M. E. & FROEHNER, S. C. (1987) Monoclonal antibody indentifies a 200-kDa subunit of the dihydropyridine-sensitive calcium channel. J. Biol. Chem. 262, 11904–7.
PAVLICA, R. J., HESLER, C. B., LIPFERT, L., HIRSHFIELD, I. N. & HALDAR, D. (1990) Two-dimensional gel electrophoretic resolution of the polypeptides of rat liver mitochondria and the outer membrane. Biochim. Biophys. Acta 1022, 115–25.
PFALLER, R., KLEENE, R. & NEUPERT, W. (1990) Biogenesis of mitochondrial porin: the import pathway. Experientia 46, 153–61.
PON, L., MOLL, T. VESTWEBER, D., MARSHALLSAY, B. & SCHATZ, G. (1989) Protein import into mitochondria: ATP-dependent protein translocation activity in a submitochondrial fraction enriched in membrane contact sites and specific proteins. J. Cell Biol. 109, 2603–16.
PUCHELLE, E., JACQUOT, J., FUCHEY, C., BURLET, H., KLOSSEK, J.-M., GILAIN, L., TRIGLIA, J.-M., THINNES, F. P. & HILSCHANN, N. (1993) Studies on human porin. IX. Immunolocalization of porin and CFTR channels in human surface respiratory epithelium. Biol. Chem. Hoppe Seyler 374, 297–304.
SAITO, A., SEILER, S., CHU, A. & FLEISCHER, S. (1984) Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J. Cell Biol. 99, 875–85.
SCHNAITMAN, C., ERWIN, V. G. & GREENAWALT, J. W. (1967) The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J. Cell. Biol. 32 719–35.
SOMMER, J. R. & WAUGH, R. A. (1976) The ultrastructure of the mammalian cardiac muscle cell-with special emphasis on the tubular membrane systems. Am. J. Pathol 82, 192–232.
THINNES, F. P. (1992) Evidence for extra-mitochondrial localization of the VDAC/Porin channel in eucaryotic cell. J. Bioenerg. Biomembr. 24, 71–5.
THINNES, F. P., FLORKE, H., WINKELBACH, H., STADT-MüLLER, U., HEIDEN, M., KARABINOS, A., HESSE, D., KRATZIN, H. D., FLEER, E. & HILSCHMANN, N. (1994) Channel active mammalian porin, purified from crude membrane fractions of human B lymphocytes or bovine skeletal muscle, reversibly binds the stilbene-disulfonate group of the chloride channel blocker DIDS. Biol Chem. Hoppe Seyler 375, 315–22.
VAUGHAN, P. C. & FRENCH, A. S. (1989) Non-ligand-activated chloride channels of skeletal muscle and epithelia. Prog. Biophys. Molec. Biol. 54, 59–79.
WINKELBACH, H., WALTER, G., MORYS-WORTMANN, C., PAETZOLD, G., HESSE, D., ZIMMERMANN, B., FLORKE, H., REYMANN, S., STADTMULLER, U. & THINNES, F. P. (1994) Studies on human porin. XII Eight monoclonal mouse anti ‘porin 31HL’ antibodies discriminate type 1 and type 2 mammalian porin channels/VDACs in western blotting and enzyme-linked immunosorbent assays. Biochem. Med. Metab. Biol. 52, 120–7.
ZALMAN, L. S., NIKAIDO, H. & KAGAWA, Y. (1980) Mitochondrial outer membrane contains a protein producing nonspecific diffusion channels. J. Biol. Chem. 255, 1771–4.
ZIZI, M., FORTE, M., BLACHLY-DYSON, E. & COLOMBINI, M. (1994) NADH regulates the gating of VDAC, the mitochondrial outer membrane channel. J. Biol. Chem. 269, 1614–16.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Junankar, P.R., Dulhunty, A.F., Curtis, S.M. et al. Porin-type1 proteins in sarcoplasmic reticulum and plasmalemma of striated muscle fibres. J Muscle Res Cell Motil 16, 595–610 (1995). https://doi.org/10.1007/BF00130241
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00130241