Abstract
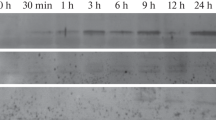
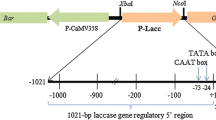
Bean leaf abscission (organ separation) correlates with thede novo accumulation of a pI 9.5 cellulase and its mRNA. Overlapping genomic clones encoding the bean abscission cellulase (BAC) were isolated and partially sequenced. In addition, a genomic clone for a soybean abscission cellulase (SAC) was identified and the sequence compared to the BAC genomic sequence. Two 5′-upstream regions are particularly well conserved in the two sequences. Of special interest here is the region between −1 and −200 in the BAC promoter which is highly conserved in the SAC gene. Particle gun bombardment with a BAC promoter construct containing 210 bp of BAC sequence 5′ to the transcription start site was sufficient to drive abscission-specific and ethylene and auxin-regulated transient expression in bean. In addition to the transient expression assay, expression was examined in stably transformed tomato. A similar −210 bp BAC promoter construct supported a low level of ethylene-inducible reporter gene expression in tomato leaf abscission zones and adjacent petioles but not in ethylene-treated stem tissue or fruit. Expression from the −210 promoter in tomato abscission zones was inhibited by silver thiosulfate, an ethylene action inhibitor, and was partially inhibited by treatment with auxin.
Similar content being viewed by others
References
Abeles FB: Role of RNA and protein synthesis in abscission. Plant Physiol 43: 1577–1586 (1968).
Abeles FB, Leather GR, Forrence LE, Craker LE: Abscission: Regulation of senescence, protein synthesis, and enzyme secretion by ethylene. HortScience 6: 371–376 (1971).
Abeles FB, Morgan PW, Saltveit ME: Ethylene in Plant Biology, 2nd ed. Academic Press, New York (1992).
Baird LM, Reid MS, Webster BD: Anatomical and physiological effects of silver thiosulfate on ethylene-induced abscission inColeus. J Plant Growth Regul 3: 217–225 (1984).
Beyer EM, Morgan PW: Abscission: the role of ethylene modification of auxin transport. Plant Physiol 48: 208–212 (1971).
Beyer EM: Abscission: the initial effect of ethylene is in the leaf blade. Plant Physiol 55: 322–327 (1975).
Chang C, Kwok SF, Bleecker AB, Meyerowitz EM:Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262: 539–544 (1993).
Cass LG, Kirven KA, Christoffersen RE: Isolation and characterization of a cellulase gene family member expressed during avocado fruit ripening. Mol Gen Genet 223: 76–86 (1990).
Chirgwin JM, Przybyla AE, MacDonald J, Rutter WJ: Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 24: 5294–5299 (1979).
Christoffersen RE, Tucker ML, Laties GG: Cellulase gene expression in ripening avocado fruit: the accumulation of cellulase mRNA and protein as demonstrated by cDNA hybridization and immunodetection. Plant Mol Biol 3: 385–391 (1984).
Cordes S, Deikman J, Margossian LJ, Fischer RL: Interaction of a developmentally regulated DNA-binding factor with sites flanking two different fruit-ripening genes from tomato. Plant Cell 1: 1025–1034 (1989).
Craker LE, Chadwick V, Leather GR: Abscission: Movement and conjugation of auxin. Plant Physiol 45: 790–793 (1970).
Deikman J, Fischer RL: Interaction of a DNA binding factor with the 5′-flanking region of an ethylene-responsive fruit ripening gene from tomato. EMBO J 7: 3315–3320 (1988).
del Campillo E, Reid PD, Sexton R, Lewis LN: Occurrence and localization of 9.5 cellulase in abscising and nonabscising tissues. Plant Cell 2: 245–254 (1990).
del Campillo E, Lewis LN: Identification and kinetics of accumulation of proteins induced by ethylene in bean abscission zones. Plant Physiol 98: 955–961 (1992).
dela Fuente RK, Leopold AC: Kinetics of abscission in the bean petiole explant. Plant Physiol 44: 251–254 (1969).
Durbin ML, Sexton R, Lewis LN: The use of immunological methods to study the activity of cellulase isozymes (β-1,4-glucan 4-glucan hydrolase) in bean leaf abscission. Plant Cell Environ 4: 67–73 (1981).
Durbin ML, Lewis LN: Cellulase inPhaseolus vulgaris. Meth Enzymol 160: 342–351 (1988).
Eyal Y, Meller Y, Lev-Yadun S, Fluhr R: A basic-type PR-1 promoter directs ethylene responsiveness, vascular and abscission zone-specific expression. Plant J 4: 225–234 (1993).
Itzhaki H, Maxson JM, Woodson WR: An ethylene-responsive enhancer element is involved in the senescence-related expression of the carnation glutathione-S-transferase (GST1) gene. Proc Natl Acad Sci USA 91: 8925–8929 (1994).
Jackson MB, Osborne DJ: Ethylene, the natural regulator of leaf abscission, Nature 225: 1019–1022 (1970).
Jefferson RA, Kavanagh TA, Bevan MW: GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO 6: 3901–3907 (1987).
Kalaitzis P, Koehler SM, Tucker ML: Cloning of a tomato polygalacturonase expressed in abscission. Plant Mol Biol 28: 647–656 (1995).
Kay R, Chan A, Daly M, McPherson J: Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 236: 1299–1302 (1987).
Kemmerer EC, Tucker ML: Comparative study of cellulases associated with adventitious root initiation, apical buds, and leaf, flower, and pod abscission zones in soybean. Plant Physiol 104: 557–562 (1994).
Kosugi S, Ohashi Y., Nakajima K, Arai I: An improved assay for β-glucuronidase in transformed cells: methanol almost completely suppresses a putative endogenous β-glucuronidase activity. Plant Sci 70: 133–140 (1990).
Lashbrook CC, Gonzalez-Bosch C, Bennett AB: Two divergent endo-β-1,4-glucanase genes exhibit overlapping expression in ripening fruit and abscising flowers. Plant Cell 6: 1485–1493 (1994).
Lewis LN, JE Varner: Synthesis of cellulase during abscission ofPhaseolus vulgaris leaf explants. Plant Physiol 46: 194–199 (1970).
Lewis LN, Linkins AE, O'Sullivan S, Reid PD: Two forms of cellulase in bean plants. In: Proceedings of the 8th International Conference of Plant Growth Substances, pp. 708–718. Hirokawa Publishing Co., Tokyo (1974).
Mattoo AK, Suttle JC: The Plant Hormone Ethylene. CRC Press, Boca Raton, FL (1991).
McCabe DE, Swain WF, Martinell BJ, Christou P: Stable transformation formation of soybean (Glycine max) by particle acceleration. Bio Technology 6: 923–926 (1988).
McCormick S, Niedermeyer J, Fry J, Barnason A, Horsch R, Fraley R: Leaf disc transformation of cultivated tomato (L. esculentum) usingAgrobacterium tumefaciens. Plant Cell Rep 5: 81–84 (1986).
Ohme-Takagi M, Shinshi H: Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7: 173–182 (1995).
Ow DW, Wood KV, DeLuca M, DeWet JR, Helinski DR, Howell SH: Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science 234: 856–859 (1986).
Riov J, Goren R: Effect of ethylene on auxin transport and metabolism in midrib sections in relation to leaf abscission of woody plants. Plant Cell Environ 2: 83–89 (1979).
Roberts JA, Taylor JE, Lasslett YV, Tucker GA: Changes in gene expression during ethylene-induced leaf abscission. In: DJ Osborne, MB Jackson (eds) Cell Separation in Plants, NATO ASI series, vol H35, pp. 61–68. Springer-Verlag, Berlin (1989).
Salinas J, Oeda K, Chua N-H: Two G-box-related sequences confer different expression patterns in transgenic tobacco. Plant Cell 4: 1485–1493 (1992).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Schardl CL, Byrd AD, Benzion G, Altschuler MA, Hildebrand DF, Hunt AG: Design and construction of a versatile system for the expression of foreign genes in plants. Gene 61: 1–11 (1987).
Silk GW, Matthews BF, Somers DA Gengenbach BG: Cloning and expression of the soybeanDapA gene encoding dihydrodipicolinate synthase. Plant Mol Biol 26: 980–993 (1994).
Sisler EC, Goren R, Huberman M: Effect of 2,5-norbornadiene on abscission and ethylere production in citrus leaf explants. Physiol Plant 63: 114–120 (1985).
Suttle JC, Hultstrand JF: Ethylene-induced leaf abscission in cotton seedlings: the physiological bases for age-dependent differences in sensitivity. Plant Physiol 95: 29–33 (1991).
Taylor JE, Tucker GA, Lasslett Y, Smith CJS, Arnold CM, Watson CF, Shuch W, Grierson D, Roberts JA: Polygalacturonase expression during leaf abscission of normal and transgenic tomato plants. Planta 183: 133–138 (1990).
Tonutti P, Cass LG, Christoffersen RE: The expression of cellulase gene family members during induced avocado fruit abscission and ripening. Plant Cell Environ 16: 709–713 (1995).
Tucker ML, Sexton R, del Campillo E, Lewis LN: Bean abscission cellulase: characterization of a cDNA clone and regulation of gene expression by ethylene and auxin. Plant Physiol 88: 1257–1262 (1988).
Tucker ML, Baird SL, Sexton R: Bean leaf abscission: tissue specific accumulation of a cellulase mRNA. Planta 186: 52–57 (1991).
Tucker ML, Milligan SB: Sequence analysis and comparison of avocado fruit and bean abscission cellulases. Plant Physiol 95: 928–933 (1991).
Zhou D, Kalaitzis P, Mattoo AK, Tucker ML: The mRNA for an ETR1 homologue in tomato is constitutively expressed in vegetative and reproductive tissues. Plant Mol Biol 30: 1331–1338 (1996).
Author information
Authors and Affiliations
Additional information
The first two authors (S.M.K. and G.L.M.) share equally in the credit and responsibility for the work presented in this article.
Rights and permissions
About this article
Cite this article
Koehler, S.M., Matters, G.L., Nath, P. et al. The gene promoter for a bean abscission cellulase is ethylene-induced in transgenic tomato and shows high sequence conservation with a soybean abscission cellulase. Plant Mol Biol 31, 595–606 (1996). https://doi.org/10.1007/BF00042232
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00042232