Abstract
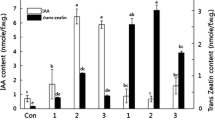
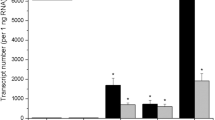
The effects of expressing a chimeric gene consisting of a soybean heat shock gene promoter and a sequence that encodes an enzyme catalyzing the synthesis of a potent phytohormone, the cytokinin iPMP, have been analyzed in transgenic tobacco plants. The production of cytokinin endogenously produced several effects previously undocumented. The differentiation of shoots independent of exogenous cytokinin from heat-treated transgenic plant leaf explants demonstrates that long-term heat treatments do not interfere with complex developmental processes. This extends the potential usefulness of heat shock gene promoters to conditionally express genes during windows of development that span several weeks.
Similar content being viewed by others
References
Ainley WM, Key JL: Development of a heat shock inducible expression cassette for plants: characterization of parameters for its use in transient expression assays. Plant Mol Biol 14: 949–967 (1990).
Ainley WM, Walker JC, Nagao RT, Key JL: Sequence and characterization of two auxin-regulated genes from soybean. J Biol Chem 263: 10658–10666 (1988).
Aloni R: The induction of vascular tissues by auxin. In: Davies PJ (ed) Plant Hormones and Their Role in Plant Growth and Development, pp. 363–374. Kluwer Academic Publishers, Boston (1988).
An G, Watson BD, Stachel S, Gordon MP, Nester EW: New cloning vehicles for transformation of higher plants. EMBO J 4: 277–284 (1985).
Buchmann I, Marner FJ, Schöder G, Waffenschmidt S, Schöder J. Tumour genes in plants: T-DNA encoded cytokinin biosynthesis. EMBO J 4: 853–859 (1985).
Cleland RE: Auxin and cell elongation. In: Davies PJ (ed) Plant Hormones and Their Role in Plant Growth and Development, pp. 132–148. Kluwer Academic Publishers, Boston (1988).
Czarnecka E, Key JL, Gurley WB: Regulatory domains of the Gmhsp 17.5-E heat shock promoter of soybean. Mol Cell Biol 9: 3457–3463 (1989).
Davies PJ: The plant hormones: their nature, occurrence, and functions. In: Davies PJ (ed) Plant Hormones and Their Role in Plant Growth and Development, pp. 1–11. Kluwer Academic Publishers, Boston (1988).
Ditta G, Stanfield S, Corbin D, Helinski DR: Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci USA 77: 7347–7351 (1980).
Figurski DH, Helinski DR. Replication of an origincontaining derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76: 1648–1652 (1979).
Fukuda H, Komamine A: Establishment of an experimental system for the study of tracheary element differentiation from single cells isolated from the mesophyll of Zinnia elegans. Plant Physiol 65: 57–60 (1980).
Gamborg OL, Miller RA, Ojima K: Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158 (1968).
Gonzàlez-Reyes A, Urquia N, Gehring WJ, Struhl G, Morata G: Are cross-regulatory interactions between homeotic genes functionally significant? Nature 344: 78–80 (1990).
Hood EE, Helmer GL, Fraley RT, Chilton M-D: The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J Bact 168: 1291–1301 (1986).
Horsch RB, Fry JE, Hoffmann NL, Wallroth M, Eichholtz D, Rogers SG, Fraley RT: A simple and general method for transferring genes into plants. Science 227: 1229–1231 (1985).
Letham DS: Zeatin, a factor inducing cell division form Zea mays. Life Sci 8: 569–573 (1963).
Letham DS, Paini LMS: The biosynthesis and metabolism of cytokinins. Annu Rev Plant Physiol 34: 163–197 (1983).
Maniatis T, Fritsch EF, Sambrook J: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1982).
McGaw BA: Cytokinin biosynthesis and metabolism. In: Davies PJ (ed) Plant Hormones and Their Role in Plant Growth and Development, pp. 76–93. Kluwer Academic Publishers, Boston (1988).
Medford JI, Horgan R, El-Sawi Z, Klee HJ: Alterations of endogenous cytokinins in transgenic plants using a chimeric isopentenyltransferase gene. Plant Cell 1: 403–413 (1989).
Metraux J-P: Gibberellins and plant cell elongation. In: Davies PJ (ed) Plant Hormones and Their Role in Plant Growth and Development, pp. 296–317. Kluwer Academic Publishers. Boston (1988).
Miller CO, Skoog F, Okomura FS, von Salza MH, Strong FM: Isolation, structure and synthesis of kinetin, a substance promoting cell division. J Am Chem Soc 78: 1345–1350 (1956).
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 (1962).
Nagao RT, Kimpel JA, Vierling E, Key JL: The heat shock response: a comparative analysis. In: Miflin BJ (ed) Oxford Surv Plant Mol Cell Biol 3, pp. 384–438. Oxford University Press, Oxford (1986).
Schreiber BMN: The role of cytokinins in maize kernel development. Ph.D. Dissertation, University of Minnesota (1990).
Schülling T, Beinsberger S, de Greef J, Schell J, van Onckelen H, Spena A. Construction of a heat-inducible chimaeric gene to increase the cytokinin content in transgenic plant tissue. FEBS Lett 249: 401–406 (1989).
Shininger TL: The control of vascular development. Annu Rev Plant Physiol 30: 313–338 (1979).
Smart CM, Scofield SR, Bevan MW, Dyer TA: Delayed leaf senescence in tobacco plants transformed with tmr, a gene for cytokinin production in Agrobacterium. Plant Cell 3: 647–656 (1991).
Smigocki AC: Cytokinin content and tissue distribution in plants transformed by a reconstructed isopentenyl transferase gene. Plant Mol Biol 16: 105–115 (1991).
Thomas H, Stoddart JL: Leaf senescence. Annu Rev Plant Physiol 31: 83–112 (1980).
Ulvskov P, Nielsen TH, Seiden P, Marcussen J: Cytokinins and leaf development in sweet pepper (Capsicum annuum L.). Planta 188: 70–77 (1992).
Vierling E. The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42: 579–620 (1991).
Vierling E, Key JL: Ribulose 1,5-bisphosphate carboxylase synthesis during heat shock. Plant Physiol 78: 155–162 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ainley, W.M., McNeil, K.J., Hill, J.W. et al. Regulatable endogenous production of cytokinins up to ‘toxic’ levels in transgenic plants and plant tissues. Plant Mol Biol 22, 13–23 (1993). https://doi.org/10.1007/BF00038992
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00038992