Abstract
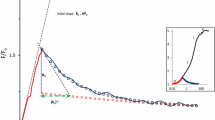
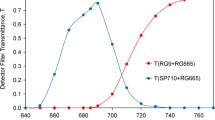
We report here the first measurements on chlorophyll (Chl) a fluorescence characteristics of photoautotrophic soybean cells (cell lines SB-P and SBI-P). The cell fluorescence is free from severe distortion problems encountered in higher plant leaves. Chl a fluorescence spectra at 77 K show, after correction for the spectral sensitivity of the photomultiplier and the emission monochromator, peaks at 688, 696 and 745 nm, representing antenna systems of photosystem II-CP43 and CP47, and photosystem I, respectively. Calculations, based on the complementary area over the Chl a fluorescence induction curve, indicated a ratio of 6 of the mobile plastoquinone (including QB) to the primary stable electron acceptor, the bound plastoquinone QA. A ratio of one between the secondary stable electron acceptor, bound plastoquinone QB, and its reduced form QB - was obtained by using a double flash technique. Owing to this ratio, the flash number dependence of the Chl a fluorescence showed a distinct period of four, implying a close relationship to the ‘S’ state of the oxygen evolution mechanism. Analysis of the QA - reoxidation kinetics showed (1) the halftime of each of the major decay components (∼ 300 μs fast and ∼ 30 ms slow) increases with the increase of diuron and atrazine concentrations; and (2) the amplitudes of the fast and the slow components change in a complementary fashion, the fast component disappearing at high concentrations of the inhibitors. This implies that the inhibitors used are able to totally displace QB. In intact soybean cells, the relative amplitude of the 30 ms to 300 μs component is higher (40:60) than that in spinach chloroplasts (30:70), implying a larger contribution of the centers with unbound QB. SB-P and SBI-P soybean cells display a slightly different sensitivity of QA - decay to inhibitors.
Similar content being viewed by others
Abbreviations
- CA:
-
complementary area over fluorescence induction curve
- Chl:
-
chlorophyll, diuron
- DCMU:
-
3-(3,4-dichlorophenyl)-1,1-dimethylurea
- F m :
-
maximum chlorophyll a fluorescence
- F 0 :
-
minimum chlorophyll a fluorescence
- F v =:
-
F t-F0
- where F v =:
-
variable chlorophyll a fluorescence
- and Ft =:
-
chlorophyll a fluorescence at time t
- PS II:
-
photosystem II
- Q a :
-
primary (plastoquinone) electron acceptor of PS II
- Q b :
-
secondary (plastoquinone) electron acceptor of PS II
- t50 :
-
the time at which the concentration of reduced Q a is 50% of that at its maximum value
References
Arnon D I (1949) Copper enzyme in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24: 1–15
Babcock G T, Blankenship R E and Sauer K (1976) Reaction kinetics for positive charge accumulation on the water side of chloroplast Photosystem II. FEBS Lett 61: 286–289
Blubaugh D J (1987) The mechanism of bicarbonate activation of plastoquinone reduction in Photosystem II of photosynthesis. PhD Thesis, Biology, University of Illinois at Urbana-Champaign
Briantais J, Vernotte C, Krause G H and Weis E (1986) Chlorophyll a fluorescence of higher plants: chloroplasts and leaves. In Govindjee, Amesz J and Ford D C (eds) Light Emission by Plants and Bacteria, pp 539–583. Orlando: Academic Press
Butler W L (1972) Primary photochemistry of Photosystem II of photosynthesis. Accnts Chem Res 6: 177–184
Crofts A R and Wraight C A (1983) The electrochemical domain of photosynthesis. Biochim Biophys Acta 726: 149–185
Delosme R (1971) New results about chlorophyll fluorescence ‘in vivo’. In Forti G, Avron M and Melandri A (eds) 2nd Int Congr Photosynthesis Res, pp 187–195. The Hague: Dr W Junk Publishers
Duysens L N M (1986) Introduction to (bacterio) chlorophyll emission: a historical perspective. In Govindjee, Amesz J and Ford D C (eds) Light Emission by Plants and Bacteria, pp 4–28. Orlando: Academic Press
Duysens L N M and Sweers H E (1963) Mechanism of two photochemical reactions in algae as studied by means of fluorescence. In Japanese Society of Plant Physiologists (eds) Studies of Microalgae and Photosynthetic Bacteria, pp 353–372. Tokyo: University of Tokyo Press
Eaton-Rye J J (1987) Bicarbonate reversible anionic inhibition of the quinone reductase in Photosystem II. PhD Thesis, Biology, University of Illinois at Urbana-Champaign
Eaton-Rye J J and Govindjee (1988) Electron transfer through the quinone acceptor complex of Photosystem II in bicarbonate depleted spinach thylakoid membranes as a function of actinic flash number and frequency. Biochim Biophys Acta, 935: 237–247
Fork D C and Mohanty P (1986) Fluorescence and other characteristics of bluegreen algae (cyanobacteria), red algae, and cryptomonads. In Govindjee, Amesz J and Fork D C (eds) Light Emission by Plants and Bacteria, pp 451–496. Orlando: Academic Press
Govindjee and Papageorgiou G C (1971) Chlorophyllfluorescence and photosynthesis: fluorescence transient. In Giese A C (ed) Photophysiology 6: 1–46, New York: Academic Press
Givindjee and Satoh K (1986) Fluorescence properties of chlorophyll b and chlorophyll c-containing algae. In Govindjee, Amesz J and Fork D C (eds) Light Emission by Plants and Bacteria, pp 497–538. Orlando: Academic Press
Green B (1988) The chlorophyll-protein complexes of higher plant photosynthetic membranes or just what green band is that? Photosynthesis Research 15: 3–32
Hodges M and Barber J (1986) Analysis of chlorophyll fluorescence induction kinetics exhibited by DCMU-inhibited thylakoids and the origin of alpha and beta centers. Biochim Biophys Acta 828: 239–246
Horn M E, Sherrard J H and Widholm J M (1983) Photoautotrophic growth of soybean cells in suspension culture. Plant Physiol 72: 426–429
Joliot A and Joliot P (1964) Étude cinetique de la reaction photochimique liberant l'oxygene au cour de la photosynthèse. CR Acad Sc Paris t 258: 4622–4625
Jursinic P and Govindjee (1977) The rise in chlorophyll a fluorescence yield and decay in delayed light emission in tris-washed chloroplasts in the 6–100 μs time range after an excitation flash. Biochim Biophys Acta 461: 253–267
Malkin S and Kok B (1966) Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yield. Biochim Biophys Acta 126: 413–432
Malkin S, Armond P A, Mooney H A and Fork D C (1981) Photosystem II photosynthetic unit sizes from fluorescence induction in leaves. Plant Physiol 67: 570–579
Mathis P and Paillotin G (1981) Primary processes of photosynthesis. In Hatch M D and Boardman N K, eds. The Biochemistry of Plants: Photosynthesis, Vol 8: pp 97–161. Sidney: Academic Press
Murashige T and Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497
Murata N and Satoh K (1986) Absorption and fluorescence emission by intact cells, chloroplasts, and chlorophyll-protein complexes. In Govindjee, Amesz J and Ford D C (eds) Light Emission by Plants and Bacteria, pp 137–159. Orlando: Academic Press
Papageorgiou G C (1975) Chlorophyll fluorescence: an intrinsic probe of photosynthesis. In Govindjee (ed) Bioenergetics of Photosynthesis, pp 319–371. New York: Academic Press
Renger G and Schreiber U (1986) Practical applications of fluorometric methods to algae and higher plant research. In Govindjee, Amesz J and Fork D C (eds) Light Emission by Plants and Bacteria, pp 587–619. Orlando: Academic Press
Robinson H H and Crofts A R (1983) Kinetics of the changes in oxidation-reduction reactions of the Photosystem II quinone acceptor complex, and the pathway for deactivation. FEBS Lett 153: 221–226
Robinson H H and Crofts A R (1987) Kinetics of the changes in oxidation-reduction states of the acceptors and donors of Photosystem II in pea thylakoids measured by flash fluorescence. In Biggins J (ed) Proceedings of the 7th International Congress of Photosynthesis, Vol 2: pp 429–432. Dordrecht: Martinus Nijhoff Publishers
Rogers S M D and Widholm J M (1986) Photosynthetic characteristics of photoautotrophic cell suspensions of soybean. Plant Physiol 80: S-46
Rogers S M D and Widholm J M (1988) Comparison of photosynthetic characteristics of two photoautotrophic cell suspension cultures of soybean. Plant Science 56: 69–74
Rogers S M D, Ogren W L and Widholm J M (1987) Photosynthetic characteristics of a photoautotrophic cell suspension culture of soybean. Plant Physiol 84: 1451–1456
Rutherford A W, Govindjee and Inoue Y (1984) Charge accumulation and photochemistry in leaves studied by thermoluminescence and delayed light emission. Proc Natl Acad Sci USA 81: 1107–1111
Sivak M N and Walker D A (1983) Some effects of CO2 concentration and decreased O2 concentration on induction of fluorescence in leaves. Proc Roy Soc London B 217: 377–392
Sivak M N, Dirtz K, Heber U and Walker D A (1985) The relationship between light scattering and chlorophyll a fluorescence during oscillations in photosynthetic carbon assimilation. Arch Biochem Biophys 237: 513–519
Sonneveld A, Rademaker H and Duysens L N M (1979) Chlorophyll a fluorescence as a monitor of nanosecond reduction of the photooxidized primary donor P-680+ of Photosystem II. Biochim Biophys Acta 548: 536–551
Taoka S, Robinson H H and Crofts A R (1983) Kinetics of the reaction of the two-electron gate of Photosystem II: studies of the competition between plastoquinone and inhibitors. In Inoue Y, Crofts A R, Govindjee, Murata N, Renger G and Satoh K (eds) The Oxygen Evolving System of Plant Photosynthesis, pp 369–381. Tokyo: Academic Press
Velthuys B R (1981) Electron-dependent competition between plastoquinone and inhibitors for binding to Photosystem II. FEBS Lett 126: 277–281
Velthuys B R and Amesz J (1974) Charge accumulation at the reducing side of system II of photosynthesis. Biochim Biophys Acta 333: 85–94
Walker D A (1981) Secondary kinetics of spinach leaves in relation to the onset of photosynthetic carbon assimilation. Planta 153: 273–278
Walker D A, Sivak M N, Prinsley R T and Cheesbrough J K (1983) Simultaneous measurement of oscillations in oxygen evolution and chlorophyll a fluorescence in leaf pieces. Plant Physiol 73: 532–549
Witt H T, Schlodder E, Brettel K and Saygin O (1986) Reaction sequences from light absorption to the cleavage of water in photosynthesis—routes, rates and intermediates. Photosynth Res 10: 453–471
Wollman F A (1978) Determination and modification of the redox state of the secondary acceptor of Photosystem II in the dark. Biochim Biophys Acta 503: 263–273
Xu C, Blair L C, Rogers S M D, Govindjee and Widholm J M (1988) Characteristics of five new photoautotrophic suspension cultures including two Amaranthus species and a Cotton strain growing on ambient CO2 levels, Plant Physiol, in press
Yamagishi A, Satoh K and Katoh S (1978) Fluorescence induction in chloroplasts isolated from the green alga Bryopsis maxima III. A fluorescence transient indicating proton gradient across the thylakoid membrane. Plant and Cell Physiol 19: 17–25
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xu, C., Rogers, S.M.D., Goldstein, C. et al. Fluorescence characteristics of photoautotrophic soybean cells. Photosynth Res 21, 93–106 (1989). https://doi.org/10.1007/BF00033363
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00033363