Abstract
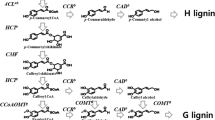
Cinnamyl alcohol dehydrogenase (CAD, EC 1.1.1.195) is an enzyme involved in lignin biosynthesis. We have previously isolated pure CAD enzyme as two closely related polypeptides of 44 and 42.5 kDa from tobacco stems. In this paper, we report partial amino acid sequences of these two polypeptides. Based on the peptide sequences mixed oligonucleotides were used to screen a tobacco stem cDNA library and CAD cDNA clones encoding the two polypeptides were identified. DNA sequence comparisons indicate very high sequence identity between these clones both in the coding and in the 5′ and 3′ untranslated sequences. The close similarity between the two CAD genes leads us to suggest that they do not represent different isoforms but are the same gene from each of the two parental lines of Nicotiana tabacum cv. Samsun. Sequence comparisons with alcohol dehydrogenase 1 (ADH1) from yeast shows sequence similarities of ca. 30%, while comparisons with maize, barley and potato ADH1 sequences show similarities of not more than 23%.
Similar content being viewed by others
Abbreviations
- CAD:
-
cinnamyl alcohol dehydrogenase (EC 1.1.1.195)
- ADH:
-
alcohol dehydrogenase (EC 1.1.1.1)
References
Aviv H, Leder P: Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid cellulose. Proc Natl Acad Sci USA 69: 1408–1412 (1969).
Birnboim HC, Doly J: A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucl Acids Res 7: 1513–1515 (1975).
Dennis ES, Gerlach WL, Pryor AJ, Benetzen JL, Inglis A, Llewellyn D, Sachs MM, Ferl RJ, Peacock WJ: Molecular analysis of the alcohol dehydrogenase ADH1 gene of maize. Nucl Acids Res 12: 3982–4000 (1984).
Edwards K, Cramer CL, Bolwell GP, Dixon R, Schuch W, Lamb CJ: Rapid transient induction of phenylalanine ammonia-lyase mRNA in elicitor-treated bean cells. Proc Natl Acad Sci USA 82: 6731–6735 (1986).
Fan F, Lerenzen JA, Plapp BV: An aspartate residue in yeast alcohol dehydrogenase I determines the specificity for coenzyme. Biochemistry 30: 6397–6401 (1991).
Gonzalez E: The C-terminal domain of plant catalases. Implications for a glyoxysomal targeting sequence. Eur J Biochem 199: 211–215 (1991).
Good A, Pelcher LE, Crosby WL: Nucleotide sequence of a complete barley ADH1 cDNA. Nucl Acids Res 16: 7182 (1988).
Grunstein M, Hogness P: Colony hybridization. A method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci USA 72: 3961–3965 (1972).
Halpin C, Knight ME, Grima-Pettenati J, Goffner D, Boudet A, Schuch W: Purification and characterisation of cinnamyl alcohol dehydrogenase from tobacco stems. Plant Physiol 98: 12–16 (1992).
Jepson I, Bray J, Jenkins G, Schuch W, Edwards K: A rapid procedure for the construction of PCR cDNA libraries from small amounts of plant tissue. Plant Mol Biol Rep 9: 131–138 (1991).
Jornvall H, Persson B, Jeffery J: Characteristics of alcohol/polyol dehydrogenases. The zinc-containing long chain alcohol dehydrogenases. Eur J Biochem 167: 195–201 (1987).
Kutsuki H, Shimada M, Higuchi T: Regulatory role of cinnamyl alcohol dehydrogenase in the formation of guaiacyl and syringyl lignins. Phytochemistry 21: 19–23 (1982).
Lee KY, Townsend J, Tepperman J, Black M, Chui CF, Mazur B, Dunsmuir P, Bedbrook J: The molecular basis of sulfonylurea herbicide resistance in tobacco. EMBO J 7: 1241–1248 (1988).
Lewis NG, Yamamoto E: Lignin: Occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol 41: 455–496 (1990).
Matton DP, Brusson N: Nucleotide sequence of two potato alcohol dehydrogenase cDNAs. Nucl Acids Res 18: 3070–3071 (1990).
Ohtsuka E, Matsuki S, Ikehara M, Takahashi Y, Matsubara K: An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem 260: 2605–2608 (1985).
Peretz M, Burstein Y: Amino acid sequence of an alcohol dehydrogenase from the thermophilic bacterium Thermoanaerobium brockii. Biochemistry 28: 6549–6555 (1989).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain termination inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Vallee BL, Auld DS: Zinc co-ordination, function and structure of zinc enzymes and other proteins. Biochemistry 29: 5647–5659 (1990).
Walter MH, Grima-Pettenati J, Grand C, Boudet AM, Lamb CJ: Cinnamyl-alcohol dehydrogenase, a molecular marker specific for lignin synthesis: cDNA cloning and mRNA induction by fungal elicitor. Proc Natl Acad Sci USA 85: 5546–5550 (1988).
Walter MH, Grima-Pettenati J, Grand C, Boudet AM, Lamb CJ: Extensive sequence similarity of the bean CAD4 (cinnamyl-alcohol dehydrogenase) to maize malic enzyme. Plant Mol Biol 15: 525–526 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Knight, M.E., Halpin, C. & Schuch, W. Identification and characterisation of cDNA clones encoding cinnamyl alcohol dehydrogenase from tobacco. Plant Mol Biol 19, 793–801 (1992). https://doi.org/10.1007/BF00027075
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00027075