Abstract
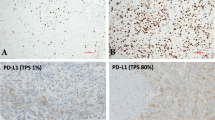
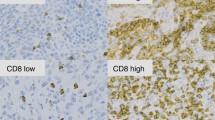
Malignant pleural mesothelioma (MPM) is a rare and aggressive form of tumour. Some mesotheliomas have been proven to be highly immunogenic. Here, we investigated the correlation between tumour infiltrating lymphocytes (TILs) or programmed cell death ligand 1 (PD-L1) expression with overall survival (OS) in patients with MPM. 62 Paraffin-embedded formalin fixed (PEFF) samples were analysed for TILs and PD-L1 expression. Patients were divided in 4 groups according to a cut-off of the percentage of TILs found per sample as measured by immunohistichemistry: “0” or absent (between 0 and 5%), “1” or low (between 6 and 25%), “2” or moderate (between 26 and 50%) and “3” or high (between 51 and 75%). OS was then correlated with different TILs’ expression patterns. Moreover, PD-L1 expression was assessed within the tumour as well as in the adjacent stroma on the same samples. Higher expression of peritumoral TILs (Group 2 + 3) versus Group 0 and 1 correlated with improved OS (p-value = 0.02). On the contrary PD-L1 expression seemed to be inversely correlated with clinical outcomes, even in the absence of statistical significance (HR 1.76; p = 0.083 95% IC 0.92–3.36 in areas within the tumour; HR 1.60; p = 0.176 95%; IC 0.80–3.19 in areas within the stroma). No relationship between TILs and PD-L1 expression was identified. Our research supports the use of TILs and PD-L1 expression as potential outcome predictors in patients with MPM. The use of TILs and PD-L1 as biomarkers for checkpoint inhibitors’ efficacy warrants future investigation.


Similar content being viewed by others
References
Robinson BW, Musk AW, Lake RA (2005) Malignant mesothelioma. Lancet 366:397–408. https://doi.org/10.1016/S0140-6736(05)67025-0
Vogelzang NJ, Rusthoven JJ, Symanowski J et al (2003) Phase III study of Pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636–2644. https://doi.org/10.1200/JCO.2003.11.136
Hassan R, Cohen SJ, Phillips M et al (2010) Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res 16:6132–6138. https://doi.org/10.1158/1078-0432.CCR-10-2275
Gralla RJ, Hollen PJ, Liepa AM, et al (2003) Improving quality of life in patients with malignant pleural mesothelioma: results of the randomized pemetrexed + cisplatin vs. cisplatin trial using the lcss-Meso instrument [abstract 2496] Proc Am Soc Clin Oncol 22
Leigh RA, Webster I (1982) Lymphocytic infiltration of pleural mesothelioma and its significance for survival. S Afr Med J 61:1007–1009
Robinson BW, Robinson C, Lake RA (2001) Localised spontaneous regression in mesothelioma—possible immunological mechanism. Lung Cancer 32:197–201
Atanackovic D, Block A, de Weerth A et al (2004) Characterization of effusion-infiltrating T cells: benign versus malignant effusions. Clin Cancer Res 10:2600–2608
DeLong P, Carroll RG, Henry AC et al (2005) Regulatory T cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer Biol Ther 4:342–346
Meloni F, Morosini M, Solari N et al (2006) Foxp3 expressing CD4+ CD25+ and CD8+ CD28− T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol 67:1–12. https://doi.org/10.1016/j.humimm.2005.11.005
Freeman GJ, Long AJ, Iwai Y et al (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027–1034
Currie AJ, Prosser A, McDonnell A et al (2009) Dual control of antitumor CD8 T cells through the programmed death-1/programmed death-ligand 1 pathway and immunosuppressive CD4 T cells: regulation and counterregulation. J Immunol 183:7898–7908. https://doi.org/10.4049/jimmunol.0901060
Mansfield AS, Roden AC, Peikert T et al (2014) B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol 9:1036–1040. https://doi.org/10.1097/JTO.0000000000000177
Cedrés S, Ponce-Aix S, Zugazagoitia J et al (2015) Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS ONE 10:e0121071. https://doi.org/10.1371/journal.pone.0121071
Yamada N, Oizumi S, Kikuchi E et al (2010) CD8 + tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol Immunother 59:1543–1549. https://doi.org/10.1007/s00262-010-0881-6
Ujiie H, Kadota K, Nitadori J-I et al (2015) The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: a comprehensive analysis reveals prognostic immune markers. Oncoimmunology 4:e1009285. https://doi.org/10.1080/2162402X.2015.1009285
Salgado R, Denkert C, Demaria S et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26:259–271. https://doi.org/10.1093/annonc/mdu450
Anraku M, Cunningham KS, Yun Z et al (2008) Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg 135:823–829. https://doi.org/10.1016/j.jtcvs.2007.10.026
Kiyotani K, Park J-H, Inoue H et al (2017) Integrated analysis of somatic mutations and immune microenvironment in malignant pleural mesothelioma. Oncoimmunology 6:e1278330. https://doi.org/10.1080/2162402X.2016.1278330
Pasello G, Zago G, Lunardi F et al (2018) Malignant pleural mesothelioma immune microenvironment and checkpoint expression: correlation with clinical–pathological features and intratumor heterogeneity over time. Ann Oncol 29:1258–1265. https://doi.org/10.1093/annonc/mdy086
Asano Y, Kashiwagi S, Goto W et al (2017) Prediction of survival after neoadjuvant chemotherapy for breast cancer by evaluation of tumor-infiltrating lymphocytes and residual cancer burden. BMC Cancer 17:888. https://doi.org/10.1186/s12885-017-3927-8
Lee HJ, Seo J-Y, Ahn J-H et al (2013) Tumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer 16:32. https://doi.org/10.4048/jbc.2013.16.1.32
Denkert C, Loibl S, Noske A et al (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28:105–113. https://doi.org/10.1200/JCO.2009.23.7370
Yamaguchi R, Tanaka M, Yano A et al (2012) Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol 43:1688–1694. https://doi.org/10.1016/j.humpath.2011.12.013
Wang K, Xu J, Zhang T, Xue D (2016) Tumor-infiltrating lymphocytes in breast cancer predict the response to chemotherapy and survival outcome: a meta-analysis. Oncotarget 7:44288–44298. https://doi.org/10.18632/oncotarget.9988
Goode EL, Block MS, Kalli KR et al (2017) Dose-response association of CD8 tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol 3:e173290. https://doi.org/10.1001/jamaoncol.2017.3290
Dong H, Strome SE, Salomao DR et al (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800. https://doi.org/10.1038/nm730
Fanoni D, Tavecchio S, Recalcati S et al (2011) New monoclonal antibodies against B-cell antigens: possible new strategies for diagnosis of primary cutaneous B-cell lymphomas. Immunol Lett 134:157–160. https://doi.org/10.1016/j.imlet.2010.09.022
Sakane T, Murase T, Okuda K et al (2018) A comparative study of PD-L1 immunohistochemical assays with four reliable antibodies in thymic carcinoma. Oncotarget 9:6993–7009. https://doi.org/10.18632/oncotarget.24075
Sobhani N, Corona SP, Bonazza D et al (2017) Advances in systemic therapy for malignant mesothelioma: future perspectives. Future Oncol 13:2083–2101. https://doi.org/10.2217/fon-2017-0224
Hassan R, Thomas A, Alewine C et al (2016) Mesothelin immunotherapy for cancer: ready for prime time? J Clin Oncol 34:4171–4179. https://doi.org/10.1200/JCO.2016.68.3672
Jahan T, Hassan R, Alley E et al (2016) Deferred publication: mesothelioma 208O_PR CRS-207 with chemotherapy (chemo) in malignant pleural mesothelioma (MPM): results from a phase 1b trial. J Thorac Oncol 11:4–156. https://doi.org/10.1016/S1556-0864(16)30330-6
Hellstrom I, Ledbetter JA, Scholler N et al (2001) CD3-mediated activation of tumor-reactive lymphocytes from patients with advanced cancer. Proc Natl Acad Sci USA 98:6783–6788. https://doi.org/10.1073/pnas.021557498
Alley EW, Lopez J, Santoro A et al (2017) Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 18:623–630. https://doi.org/10.1016/S1470-2045(17)30169-9
Quispel-Janssen J, van der Noort V, de Vries JF et al (2018) PD-1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol. https://doi.org/10.1016/j.jtho.2018.05.038
Fennell DA, Kirkpatrick E, Cozens K et al (2018) CONFIRM: a double-blind, placebo-controlled phase III clinical trial investigating the effect of nivolumab in patients with relapsed mesothelioma: study protocol for a randomised controlled trial. Trials 19:233. https://doi.org/10.1186/s13063-018-2602-y
Desai A, Karrison T, Rose B et al (2018) Phase II trial of pembrolizumab (P) in patients (pts) with previously-treated mesothelioma (MM). J Clin Oncol 36:8565–8565. https://doi.org/10.1200/JCO.2018.36.15_suppl.8565
Thapa B, Salcedo A, Lin X et al (2017) The immune microenvironment, genome-wide copy number aberrations, and survival in mesothelioma. J Thorac Oncol 12:850–859. https://doi.org/10.1016/j.jtho.2017.02.013
Lantuejoul S, Le Stang N, Damiola F et al (2017) PD-L1 testing for immune checkpoint inhibitors in mesothelioma: for want of anything better? J Thorac Oncol 12:778–781. https://doi.org/10.1016/j.jtho.2017.03.018
Acknowledgements
Supported by Fondazione “Aldo Duca” and Progetto FRA 2016–2017, Dipartimento Universitario Clinico di Scienze Mediche, Chirurgiche e della Salute, Università degli Studi di Trieste.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent for publication was obtained from all authors of this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sobhani, N., Roviello, G., Pivetta, T. et al. Tumour infiltrating lymphocytes and PD-L1 expression as potential predictors of outcome in patients with malignant pleural mesothelioma. Mol Biol Rep 46, 2713–2720 (2019). https://doi.org/10.1007/s11033-019-04715-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04715-9