Abstract
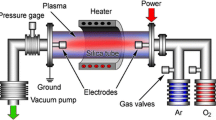
In this paper, we show experimental evidence about the growth mechanism of one-dimensional ZnS nanostructures through electrophoretic deposition. ZnS nanoparticles with 20 nm of mean diameter were prepared by microwave-assisted synthesis using sodium citrate as the stabilizer. The resulting aqueous dispersion was deposited without any further preparation by means of electrophoretic methods using aluminum plates as electrodes under 600 mV in order to avoid bubble formation. FE-SEM images of different deposition times confirm the previously proposed mechanism of lyosphere distortion and thinning and the subsequent dipole–dipole interactions’ phenomena where the incoming particles are oriented by the particles previously deposited. In this mechanism, the first particle/lyosphere deposited acts as a dipole and attract the incoming particles to it, creating a one-dimensional nanostructure after some time of deposition. These results can help understand the different mechanisms of the electrophoretic deposition and are useful for future nanostructure designs of semiconductor materials.





Similar content being viewed by others
Abbreviations
- EPD:
-
Electrophoretic deposition
- FE-SEM:
-
Field emission scanning electron microscopy
- MW:
-
Microwave
- TEM:
-
Transmission electron microscopy
- HRTEM:
-
High resolution transmission electron microscopy
References
Yang PD, Yan RX, Fardy M (2010) Nano Lett 10:1529. doi:10.1021/nl100665r
Zhang XT, Liu Z, Li Q, Hark SK (2005) J Phys Chem B 109:17913. doi:10.1021/jp0527406
Height MJ, Madler L, Pratsinis SE, Krumeich F (2006) Chem Mater 18:572. doi:10.1021/cm052163y
Fortuna SA, Li XL (2010) Semicond Sci Technol 25(2):024005. doi:10.1088/0268-1242/25/2/024005
Ho ST, Chen KC, Chen HA, Lin HY, Cheng CY, Lin HN (2007) Chem Mater 19:4083. doi:10.1021/cm070474y
Jie JS, Zhang WJ, Bello I, Lee CS, Lee ST (2010) Nano Today 5:313. doi:10.1016/j.nantod.2010.06.009
Shen GZ, Bando Y, Golberg D, Zhou CW (2008) J Phys Chem C 112:12299. doi:10.1021/jp8039687
Barrelet CJ, Wu Y, Bell DC, Lieber CM (2003) J Am Chem Soc 125:11498. doi:10.1021/ja036990g
Boccaccini AR, Roether JA, Thomas BJC, Shaffer MSP, Chavez E, Stoll E, Minay EJ (2006) J Ceram Soc Jpn 114:1. doi:10.2109/jcersj.114.1
Mohammadi MR, Ordikhani F, Fray DJ, Khomamizadeh F (2011) Particuology 9:161. doi:10.1016/j.partic.2010.07.026
Wang HW, Ting CF, Hung MK, Chiou CH, Liu YL, Liu ZW, Ratinac KR, Ringer SP (2009) Nanotechnology 20(5):055601. doi:10.1088/0957-4484/20/5/055601
Cao GZ (2004) J Phys Chem B 108:19921. doi:10.1021/jp040492s
Vázquez A, López I, Gómez I (2011) Mater Lett 65:2422. doi:10.1016/j.matlet.2011.04.107
Trindade T, O’Brien P, Pickett NL (2001) Chem Mater 13:3843. doi:10.1021/cm000843p
Tiemann M, Marlow F, Brieler F, Linden M (2006) J Phys Chem B 110:23142. doi:10.1021/jp0638383
Yu JH, Joo J, Park HM, Baik SI, Kim YW, Kim SC, Hyeon T (2005) J Am Chem Soc 127:5662. doi:10.1021/ja044593f
Sarkar P, Nicholson PS (1996) J Am Ceram Soc 79:1987. doi:10.1111/j.1151-2916.1996.tb08929.x
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vázquez, A., López, I.A. & Gómez, I. Growth mechanism of one-dimensional zinc sulfide nanostructures through electrophoretic deposition. J Mater Sci 48, 2701–2704 (2013). https://doi.org/10.1007/s10853-012-7066-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-7066-y