Abstract
Introduction and aim
NfL and GFAP are promising blood-based biomarkers for Alzheimer's disease. However, few studies have explored plasma GFAP in the prodromal and preclinical stages of AD. In our cross-sectional study, our aim is to investigate the role of these biomarkers in the earliest stages of AD.
Materials and methods
We enrolled 40 patients (11 SCD, 21 MCI, 8 AD dementia). All patients underwent neurological and neuropsychological examinations, analysis of CSF biomarkers (Aβ42, Aβ42/Aβ40, p-tau, t-tau), Apolipoprotein E (APOE) genotype analysis and measurement of plasma GFAP and NfL concentrations. Patients were categorized according to the ATN system as follows: normal AD biomarkers (NB), carriers of non-Alzheimer's pathology (non-AD), prodromal AD, or AD with dementia (AD-D).
Results
GFAP was lower in NB compared to prodromal AD (p = 0.003, d = 1.463) and AD-D (p = 0.002, d = 1.695). NfL was lower in NB patients than in AD-D (p = 0.011, d = 1.474). NfL demonstrated fair accuracy (AUC = 0.718) in differentiating between NB and prodromal AD, with a cut-off value of 11.65 pg/mL. GFAP showed excellent accuracy in differentiating NB from prodromal AD (AUC = 0.901) with a cut-off level of 198.13 pg/mL.
Conclusions
GFAP exhibited excellent accuracy in distinguishing patients with normal CSF biomarkers from those with prodromal AD. Our results support the use of this peripheral biomarker for detecting AD in patients with subjective and objective cognitive decline.
Similar content being viewed by others
Introduction
Research and clinical practice on Alzheimer's disease (AD) are at a turning point. The Food and Drug Administration (FDA) has provisionally approved two anti-amyloid monoclonal antibodies for the treatment of patients with mild cognitive impairment (MCI) due to AD and mild AD dementia [1]. This represents a significant milestone as these are the first disease-modifying therapies for AD. Consequently, neurologists, researchers, and health services will face increasing demands for diagnostic assessments of patients with cognitive disorders, as well as the need to diagnose AD at its earliest stages to halt the pathological process before neurodegeneration begins [2]. Currently used disease biomarkers, such as cerebrospinal fluid (CSF) biomarkers [3, 4], PET neuroimaging, and brain MRI [5,6,7], are highly accurate in detecting AD pathology. However, their application on large populations is severely limited due to cost, limited accessibility, and invasiveness.
Blood-based biomarkers are considered promising tools that could overcome these limitations and enable biomarker assessments even at the primary care level, where most individuals with cognitive symptoms present [8]. Among the peripheral biomarkers, Neurofilament light chain (NfL) and Glial fibrillary acidic protein (GFAP) are emerging as reliable biomarkers of AD, being associated with CSF biomarkers [9,10,11]. NfL is a component of the neuronal cytoskeleton and is released into the cerebrospinal fluid and blood following neuronal damage [12]. GFAP is a marker of reactive astrogliosis [13], associated with morphological, molecular, and functional remodeling of astrocytes surrounding Aβ plaques [14]. Elevated levels of NfL and GFAP have been observed in subjective cognitive decline (SCD), MCI and AD dementia [15,16,17]. However, the accuracy of plasma NfL and GFAP in predicting underlying AD pathology in patients with SCD and MCI has been poorly explored so far [10, 18,19,20]. Therefore, our aim is to assess the accuracy of plasma NfL and GFAP in predicting AD pathology in SCD and MCI patients.
Materials and Methods
Study design, patients, and assessments
This is a cross-sectional analysis involving 40 consecutive patients (11 patients with SCD, 21 patients with MCI, and 8 patients with AD dementia) referred to the Centre for Alzheimer’s Disease and Adult Cognitive Disorders of Careggi Hospital in Florence for assessment of cognitive decline, since July 2018 to November 2022. We included patients that met the following criteria: (1) clinical diagnosis of AD dementia [21], (2) clinical diagnosis of MCI [22], (3) clinical diagnosis of SCD [23]. Exclusion criteria were: history of head injury, current systemic and/or neurological disease other than AD, major depression, or substance use disorder. All patients underwent: comprehensive clinical assessment, neurological examination; extensive neuropsychological investigation and brain MRI or CT scan; 18F-Fluorodeoxyglucose-PET brain scan (18F-FDG-PET); blood collection for Apolipoprotein E (APOE) genotype analysis and measurement of plasma NfL and GFAP concentration; CSF collection for Aβ42, Aβ42/Aβ40, total-tau (t-tau) and phosphorylated-tau (p-tau) measurement. We defined age at baseline as the age at the time of plasma collection, disease duration as the time from the onset of symptoms to baseline examination, and positive family history of dementia if one or more first-degree relatives were reported to have documented cognitive decline. We defined disease duration as the timeframe from the onset of symptoms to baseline examination and family positive history of dementia if one or more first-degree relatives were reported to have documented cognitive decline.
Neuropsychological assessment
All subjects were evaluated by an extensive neuropsychological battery including: global cognition measure (MMSE) [24], tasks exploring verbal and spatial short- working and long-term memory (Digit and Visuo-spatial Span forward and backward [25], Rey Auditory Verbal Learning Test – RAVLT [26], Short Story Immediate and Delayed Recall [27], Rey-Osterrieth complex figure recall [28]), attention (Trail Making Test A, attentional matrices [29]), language (Category Fluency Task [30] and Phonemic Fluency Task [26]), constructional praxis (Rey-Osterrieth complex figure copy) and executive function (Trail Making Test B [31], Stroop Test [32]). In SCD patients, cognitive complaints were explored at baseline using a survey based on the Memory Assessment Clinics-Questionnaire [33].
Plasma GFAP and NfL analysis
Blood was collected by venipuncture into standard polypropylene EDTA test tubes (Sarstedt, Nümbrecht, Germany) and centrifuged within two hours at 1300 rcf at room temperature for 10 min. Plasma was isolated and stored at -80 °C until testing. Plasma biomarkers analysis was performed with Simoa NF-Light SR-X kit (cat. No 103400) and with Simoa GFAP SR-X kit (cat. No 102336) for human samples provided by Quanterix Corporation (Lexington, MA, USA) on the automatized Simoa SR-X platform (GBIO, Hangzhou, China), following the manufacturer’s instructions [34]. The Lower Limit of Quantification (LLOQ) and the Limit of Detection (LOD) provided by the NF-Light kit were 0.316 pg/mL and 0.0552 pg/mL, respectively. The LLOQ and the LOD of GFAP kit were 1.37 pg/mL and 0.26 pg/mL, respectively. Plasma NfL and GFAP concentrations of all samples were detected in a single run basis. Quality controls (low NfL concentration = 5.08 pg/mL and high NfL concentration = 169 pg/mL; low GFAP concentration = 48.0 pg/mL and high GFAP concentration = 1063 pg/mL) were included in the array and tested with samples. A calibration curve was determined from measurements of serially diluted calibrators provided by Quanterix. Plasma samples and controls were diluted at a 1:4 ratio and measured in duplicate with calibrators.
APOE ε4 genotyping
A standard automated method (QIAcube, QIAGEN) was used to isolate DNA from peripheral blood samples. APOE genotypes were investigated by high-resolution melting analysis (HRMA)[35]. Two sets of PCR primers were designed to amplify the regions encompassing rs7412 [NC_000019.9:g[M13] [GG14] 0.45412079C > T] and rs429358 (NC_000019.9:g.45411941 T > C). The samples with known APOE genotypes, which had been validated by DNA sequencing, were used as standard references.
CSF Collection and biomarkers analysis
CSF was collected at 8.00 a.m. by lumbar puncture, immediately centrifuged and stored at -80 °C until performing the analysis. Aβ42, Aβ42/ Aβ40 ratio, t-tau, and p-tau were measured using a chemiluminescent enzyme immunoassay (CLEIA) analyzer LUMIPULSE G600 (Fujirebio). Cut-offs for normal values were: for Aβ42 > 670 pg/ml, Aβ42/Aβ40 ratio > 0.062, t-tau < 400 pg/ml and p-tau < 60 pg/ml [36].
Brain 18F-FDG-PET acquisition and rating
18F-FDG-PET scans were acquired following the EANM procedure guidelines [37], using an advanced hybrid PET-CT scanner in 3D list mode. PET data were reconstructed using 3D iterative algorithm, corrected for attenuation, random and scatter using the manufacturer’s software. A trained nuclear medicine physician visually rated all scans as positive or negative, according to the European Association of Nuclear Medicine and European Academy of Neurology recommendations [38], as described in a previous work [39].
Classification of patients according to the ATN classification
Based on biomarker results, patients were classified according to the NIA-AA Research Framework [40]: patients were rated as A + if at least one of the amyloid biomarkers (Aβ42 or Aβ42/ Aβ40 ratio) revealed the presence of Aβ pathology, and as A- if none of the biomarkers revealed the presence of Aβ pathology. Patients were classified as T + or T- if CSF p-tau concentrations were higher or lower than the cut-off value, respectively. Patients were classified as N + if at least one neurodegeneration biomarker was positive (CSF t-tau higher than the cut-off value or positive 18F-FDG-PET). In the case of discordant results between CSF and 18F-FDG-PET, we considered only the pathologic result. Based on this first classification, considering our sample size and to avoid creating excessively small groups, we classified patients as follows: i) carriers of normal biomarkers (NB) if all the biomarkers were negative (A-/T-/N-); ii) non Alzheimer’s pathologic change (non-AD) if they had positive p-tau and/or t-tau (A-/T + /N-, A-/T + /N-, A-/T + /N +); iii) prodromal AD (SCD and MCI) or AD-dementia (AD) if they had positive amyloid biomarkers and positive p-tau; we included in this group also patients with isolated Aβ pathology (A + /T-/N-, A + /T + /N-, A + /T + /N +).
Statistical analysis
All statistical analyses were performed via IBM SPSS Statistics Software Version 25 (SPSS Inc., Chicago, USA) and the computing environment R 4.2.3 (R Foundation for Statistical Computing, Vienna, 2013). Figures were created using R 4.2.3 and Adobe Illustrator (Adobe Inc., San Jose, California). Statistical significance received Bonferroni adjustment for multiple comparisons being accepted at p < 0.005. Distributions of all variables were assessed through the Shapiro–Wilk test. As NfL and GFAP were not normally distributed, we applied a log10 transformation. This transformation resulted in normally distributed data that met the assumptions of parametric statistical tests that were necessary to evaluate our hypotheses. We conducted descriptive statistics to examine the central tendency and variability of our data using means and standard deviations (SD) for continuous variables and frequencies or percentages and 95% confidence interval (95% CI) for categorical variables, respectively. We used t-test for comparison between two groups, one-way analysis of variance (ANOVA) with Bonferroni post-hoc test for comparison between three or more groups, Pearson’s correlation coefficient to evaluate correlations between groups’ numeric measures and chi-square test to compare categorical data. To adjust for possible confounding factors, we used analysis of covariance (ANCOVA). We calculated the size effect by Cohen’s d for normally distributed numeric measures, η2 for ANOVA and Cramer’s V for categorical data. We constructed receiver operating characteristic (ROC) curves and calculated the area under the curve (AUC) to evaluate the performance of plasma NfL and GFAP to predict AD. We used the maximize metric method to determine the optimal cut-off value for NfL and GFAP and calculated accuracy, sensitivity, and specificity.
Results
Description of the groups
Sixteen patients (40.00%, 8 SCD and 8 MCI) were classified as NB, four patients (10.00%, 1 SCD and 3 MCI) were rated as non-AD, 12 patients (30.00%, 2 SCD and 10 MCI) were rated as prodromal AD, including three A + /T-/N-. All eight patients with clinical diagnosis of AD showed biomarkers consistent with AD (6 A + /T + /N + and 2 A + /T-/N +) and were defined as AD-dementia. Thirteen patients (32.50%) had positive 18F-FDG-PET, including five patients with prodromal AD (1 SCD and 4 MCI) and all the eight AD-dementia patients. Negative 18F-FDG-PET scans showed normal brain metabolism and were not suggestive for any other neurological conditions. A detailed description of distributions of A/T/N subtypes among groups (SCD, MCI and AD-dementia) is reported in Table 1.
Patients in the NB group were younger than non-AD (p = 0.001, d = 2.448) and had higher MMSE compared to prodromal AD (p = 0.002, d = 1.576) and AD-dementia (p < 0.001, d = 2.042). There were no differences in gender, years of education or APOE frequencies between NB, non-AD, prodromal AD and AD-dementia (Table 2).
Comparisons of CSF and blood biomarkers between groups
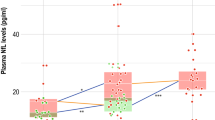
CSF and blood biomarkers levels were different between groups: Aβ42 (F [3, 36] = 20.7, p < 0.001, η2 = 0.633), Aβ42/Aβ40 (F [3, 36] = 20.7, p < 0.001, η2 = 0.740), p-tau (F [3, 36] = 8.63, p < 0.001, η2 = 0.418), t-tau (F [3, 36] = 8.87, p < 0.001, η2 = 0.425) and LogGFAP (F [3, 36] = 7.65, p < 0.001, η2 = 0.389). As expected, post-hoc analysis showed that Aβ42 concentration and Aβ42/Aβ40 ratio were higher in NB compared to prodromal AD (p < 0.001, d = 1.901; p < 0.001, d = 3.330, respectively) and AD with dementia (p < 0.001, d = 2.39; p < 0.001, d = 3.269, respectively) as well as in non-AD compared to prodromal AD (p < 0.001, d = 3.193; p < 0.001, d = 2.706, respectively) and AD with dementia (p < 0.001, d = 3.684; p < 0.001, d = 2.645, respectively). On the opposite, p-tau and t-tau were lower in NB compared to prodromal AD (p < 0.001, d = 1.689; p < 0.001, d = 1.658, respectively) and AD with dementia (p = 0.002, d 0 1.731; p < 0.001, d = 1.812, respectively). There were no differences in p-tau and t-tau between non-AD, prodromal AD and AD dementia. LogGFAP was lower in NB compared to prodromal AD (p = 0.003, d = 1.463) and AD with dementia (p = 0.002, d = 1.695). LogNfL was lower in NB patients than in AD-dementia (p = 0.004, d = 1.474) (Fig. 1). There were no differences in CSF and plasma biomarkers among non-AD, prodromal AD and AD-dementia. There were no differences in plasma NfL and GFAP concentration between males and females (p = 0.737, η2 = 0.003; p = 0.64, η2 = 0.006, respectively) or between APOE ε4 + and APOE ε4- (p = 0.908, η2 < 0.001; p = 0.376, η2 = 0.023, respectively) (Table 2).
Plasma NFL and GFAP across groups. Values quoted in the y-axis indicate LogNfL and LogGFAP levels. Horizontal bars at the top indicate significant differences between groups. Horizontal bars superimposed on the violin plots indicate mean and SD. A. NB vs. AD-dementia (p = 0.004, d = 1.474). B. NB vs prodromal AD (p = 0.003, d = 1. 463); NB vs. AD-dementia (p = 0.002, d = 1.695)
Accuracy of plasma NfL and GFAP in predicting AD
We aimed to estimate the performances of plasma NfL and GFAP in differentiating patients with SCD and MCI who were carriers of positive AD biomarkers from patients who were not. Therefore, for this analysis we considered patients in the NB group and prodromal AD patients. We did not consider non-AD, due to the small number of patients, or AD-dementia patients, as we were not interested in differentiating patients with full-blown dementia from patients with SCD or MCI. NfL showed a fair accuracy (AUC = 0.718, accuracy = 70.37% [95% C.I. = 53.46: 87.28], sensitivity = 75.00% [95% C.I. = 58.96: 91.04]), specificity = 66.67 [95% C.I. = 49.21: 84.13], PPV = 61.54% [95% C.I. = 42.84: 80.24], NPV = 76.92 [95% C.I. = 60.73: 93.12]) in differentiating between NB and prodromal AD, with a cut-off value of 11.65 pg/mL. GFAP showed an excellent accuracy in differentiating NB from prodromal AD (AUC = 0.901, accuracy = 85.71% [95% C.I. = 72.75: 98.67], sensitivity = 66.67 [95% C.I. = 49.21: 84.13], specificity = 100%, PPV = 100%, NPV = 80.00 [95% C.I. = 65.18: 94.82]) with a cut-off level of 198.13 pg/mL (Fig. 2).
Discussion
Plasma GFAP was highly accurate in predicting AD in patients with subjective symptoms or objective signs of cognitive decline, in line with the emerging literature [17]. The mean plasma GFAP concentration in our sample was consistent with previous reports on larger samples, but higher compared to Cicognola et al. [41]. Cicognola et al. also suggested a lower cut-off value (44 pg/mL) than the one we identified, despite using the same analysis methods for plasma NfL and GFAP. On the other hand, our cut-off value is slightly lower than the one identified by Oeckl et al. [42] (245 pg/mL) to differentiate patients with AD from healthy controls. This discrepancy may be explained by the fact that we considered patients with subjective cognitive decline (SCD) and mild cognitive impairment (MCI), while we did not include patients with AD dementia in the accuracy analysis.
Plasma NfL showed fair accuracy in discriminating between patients with normal biomarkers and patients with prodromal AD. These results are consistent with previous studies that indicated blood GFAP as more accurate than blood NfL. Nevertheless, in a previous study [43], we demonstrated that plasma NfL concentration was higher in patients with AD pathology (defined as A + /T + /N + or A + /T + /N- according to Jack et al. [2]) compared to patients with normal AD biomarkers and isolated Aβ pathology. We also showed that NfL concentrations were similar between patients with normal AD biomarkers and patients with isolated Aβ pathology. Therefore, based on our results, we can speculate that GFAP and NfL provide different information regarding the Alzheimer's continuum. GFAP seems to predict the presence of Aβ pathology regardless of tauopathy and neurodegeneration, while NfL might be more accurate in discriminating patients who also developed tauopathy, as supported by other authors [44]. This might also reflect previous findings showing that reactive astrocytosis is a very early process associated with Alzheimer’s pathology, preceding both amyloid plaque deposition and neurodegeneration [45, 46]. Consequently, we might suggest different roles for GFAP and NfL, with GFAP being more informative in the earlies stages of the disease and NfL being more useful in monitoring the progression of Alzheimer's pathological changes to Alzheimer's disease.
The main limitation of our study is the small sample size, particularly when we split the sample according to the ATN classification. Another limitation is that we did not include a sample of healthy control individuals. Additionally, being a single-center study, there may be biases related to assessment and diagnosis procedures. However, we would like to highlight some novelties of our work that may provide useful evidence for both clinical practice and AD research. While many studies have demonstrated the potential of NfL and GFAP, most of them have focused on predicting progression from MCI to dementia [19, 47, 48], whereas only a few studies have investigated the pathologic substrate of MCI defined according to AD biomarkers [19, 42, 44]. As the definition of AD has shifted from a purely clinical entity to a clinic-biological construct based on biomarker profiles, understanding the relationship between blood-based biomarkers and CSF biomarker profiles can have significant clinical implications for early AD diagnosis. Thus, we considered the presence of AD pathology as the outcome, biologically defined through CSF biomarkers, which is a strength of our study. Additionally, unlike previous larger studies that classified patients based on Aβ pathology [18, 19, 44, 47], we also considered tau pathology and neurodegeneration biomarkers, providing a comprehensive evaluation of plasma NfL and GFAP concentrations associated with different biomarker profiles. This is relevant as each biomarker profile has been associated with a different risk of progression to dementia. Moreover, only a few studies have included patients with SCD in their investigations of NfL, and we are aware of only one other study that assessed GFAP in SCD patients. SCD represents a clearly defined clinical entity with a higher risk of AD and progression to dementia compared to cognitively healthy individuals [49,50,51,52]. Based on this evidence, the National Institute of Aging-Alzheimer’s Association (NIA-AA) included SCD as the first manifestation of the symptomatic stages of AD preceding MCI. This distinction is relevant for the future application of blood-based biomarkers in clinical practice, particularly as disease-modifying treatments become available. It is widely understood that DMTs should be administered at the earliest stages of the disease to halt the pathological process before neurodegeneration begins [53]. However, general population screening may lead to an unacceptable number of false positive results and subsequent costs. In this perspective, patients with SCD represent an optimal selected population to be screened for prodromal AD. Our results provide one of the first pieces of evidence regarding plasma GFAP in this group of patients.
In conclusion, our work offers further insights into the utility of blood-based biomarkers in the prodromal phase of AD. Specifically, our results support the use of blood-based biomarkers in predicting Alzheimer’s pathology in patients with SCD and MCI, which represents a promising tool for biomarker assessment. This tool can also be applied at the primary care level to assist clinicians in determining the most appropriate and personalized assessment pathway for each patient.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rabinovici GD (2021) Controversy and Progress in Alzheimer’s Disease - FDA Approval of Aducanumab. N Engl J Med 385:771–774. https://doi.org/10.1056/NEJMp2111320
Jack CR, Bennett DA, Blennow K et al (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14:535–562. https://doi.org/10.1016/j.jalz.2018.02.018
Lewczuk P, Esselmann H, Otto M et al (2004) Neurochemical diagnosis of Alzheimer’s dementia by CSF Abeta42, Abeta42/Abeta40 ratio and total tau. Neurobiol Aging 25:273–281. https://doi.org/10.1016/S0197-4580(03)00086-1
Buerger K, Ewers M, Pirttilä T et al (2006) CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 129:3035–3041. https://doi.org/10.1093/brain/awl269
Clark CM, Pontecorvo MJ, Beach TG et al (2012) Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol 11:669–678. https://doi.org/10.1016/S1474-4422(12)70142-4
Sabri O, Sabbagh MN, Seibyl J et al (2015) Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: phase 3 study. Alzheimers Dement 11:964–974. https://doi.org/10.1016/j.jalz.2015.02.004
Mueller A, Bullich S, Barret O et al (2020) Tau PET imaging with 18F-PI-2620 in Patients with Alzheimer Disease and Healthy Controls: A First-in-Humans Study. J Nucl Med 61:911–919. https://doi.org/10.2967/jnumed.119.236224
Hansson O, Edelmayer RM, Boxer AL et al (2022) The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimer’s & Dementia 18:2669–2686. https://doi.org/10.1002/alz.12756
Hansson O, Blennow K, Zetterberg H, Dage J (2023) Blood biomarkers for Alzheimer’s disease in clinical practice and trials. Nat Aging 3:506–519. https://doi.org/10.1038/s43587-023-00403-3
Ebenau JL, Pelkmans W, Verberk IMW et al (2022) Association of CSF, Plasma, and Imaging Markers of Neurodegeneration With Clinical Progression in People With Subjective Cognitive Decline. Neurology 98:e1315–e1326. https://doi.org/10.1212/WNL.0000000000200035
Baiardi S, Quadalti C, Mammana A et al (2022) Diagnostic value of plasma p-tau181, NfL, and GFAP in a clinical setting cohort of prevalent neurodegenerative dementias. Alzheimers Res Ther 14:153. https://doi.org/10.1186/s13195-022-01093-6
Gafson AR, Barthélemy NR, Bomont P et al (2020) Neurofilaments: neurobiological foundations for biomarker applications. Brain 143:1975–1998. https://doi.org/10.1093/brain/awaa098
Escartin C, Galea E, Lakatos A et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24:312–325. https://doi.org/10.1038/s41593-020-00783-4
Osborn LM, Kamphuis W, Wadman WJ, Hol EM (2016) Astrogliosis: An integral player in the pathogenesis of Alzheimer’s disease. Prog Neurobiol 144:121–141. https://doi.org/10.1016/j.pneurobio.2016.01.001
Mattsson N, Andreasson U, Zetterberg H et al (2017) Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol 74:557–566. https://doi.org/10.1001/jamaneurol.2016.6117
Benedet AL, Milà-Alomà M, Vrillon A et al (2021) Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum. JAMA Neurol 78:1–13. https://doi.org/10.1001/jamaneurol.2021.3671
Shen X-N, Huang S-Y, Cui M et al (2023) Plasma Glial Fibrillary Acidic Protein in the Alzheimer Disease Continuum: Relationship to Other Biomarkers, Differential Diagnosis, and Prediction of Clinical Progression. Clin Chem 69:411–421. https://doi.org/10.1093/clinchem/hvad018
Tosun D, Veitch D, Aisen P et al (2021) Detection of β-amyloid positivity in Alzheimer’s Disease Neuroimaging Initiative participants with demographics, cognition. MRI and plasma biomarkers. Brain Commun 3:fcab008. https://doi.org/10.1093/braincomms/fcab008
Chatterjee P, Pedrini S, Ashton NJ et al (2022) Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer’s disease. Alzheimer’s & Dementia 18:1141–1154. https://doi.org/10.1002/alz.12447
Giacomucci G, Mazzeo S, Bagnoli S et al (2022) Plasma neurofilament light chain as a biomarker of Alzheimer’s disease in Subjective Cognitive Decline and Mild Cognitive Impairment. J Neurol 269:4270–4280. https://doi.org/10.1007/s00415-022-11055-5
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Albert MS, DeKosky ST, Dickson D et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:270–279. https://doi.org/10.1016/j.jalz.2011.03.008
Jessen F, Amariglio RE, van Boxtel M et al (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10:844–852. https://doi.org/10.1016/j.jalz.2014.01.001
Magni E, Binetti G, Bianchetti A et al (1996) Mini-Mental State Examination: a normative study in Italian elderly population. Eur J Neurol 3:198–202. https://doi.org/10.1111/j.1468-1331.1996.tb00423.x
Monaco M, Costa A, Caltagirone C, Carlesimo GA (2013) Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an Italian adult population. Neurol Sci 34:749–754. https://doi.org/10.1007/s10072-012-1130-x
Carlesimo GA, Caltagirone C, Gainotti G (1996) The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol 36:378–384. https://doi.org/10.1159/000117297
De Renzi E, Faglioni P, Ruggerini C (1977) Prove di memoria verbale di impiego clinico per la diagnosi di amnesia. Archivio di Psicologia, Neurologia, Psichiatria 38:303–318
Caffarra P, Vezzadini G, Dieci F et al (2002) Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurol Sci 22:443–447. https://doi.org/10.1007/s100720200003
Della Sala S, Laiacona M, Spinnler H, Ubezio C (1992) A cancellation test: its reliability in assessing attentional deficits in Alzheimer’s disease. Psychol Med 22:885–901. https://doi.org/10.1017/s0033291700038460
Novelli G, Papagno C, Capitani E, Laiacona M (1986) Tre test clinici di ricerca e produzione lessicale. Taratura su sogetti normali. / Three clinical tests to research and rate the lexical performance of normal subjects. Archivio di Psicologia, Neurologia e Psichiatria 47(4):477–506
Giovagnoli AR, Del Pesce M, Mascheroni S et al (1996) Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci 17:305–309. https://doi.org/10.1007/BF01997792
Caffarra P, Vezzadini G, Dieci F et al (2002) Una versione abbreviata del test di Stroop: Dati normativi nella popolazione Italiana. Riv Neurol 12:111–115
Crook TH, Feher EP, Larrabee GJ (1992) Assessment of memory complaint in age-associated memory impairment: the MAC-Q. Int Psychogeriatr 4:165–176. https://doi.org/10.1017/s1041610292000991
Rissin DM, Kan CW, Campbell TG et al (2010) Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 28:595–599. https://doi.org/10.1038/nbt.1641
Sorbi S, Nacmias B, Forleo P et al (1994) ApoE allele frequencies in Italian sporadic and familial Alzheimer’s disease. Neurosci Lett 177:100–102. https://doi.org/10.1016/0304-3940(94)90054-x
Alcolea D, Pegueroles J, Muñoz L et al (2019) Agreement of amyloid PET and CSF biomarkers for Alzheimer’s disease on Lumipulse. Annals of Clinical and Translational Neurology 6:1815–1824. https://doi.org/10.1002/acn3.50873
Varrone A, Asenbaum S, Vander Borght T et al (2009) EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging 36:2103–2110. https://doi.org/10.1007/s00259-009-1264-0
Nobili F, Arbizu J, Bouwman F et al (2018) European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain 18F-fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: Delphi consensus. Eur J Neurol 25:1201–1217. https://doi.org/10.1111/ene.13728
Giacomucci G, Mazzeo S, Bagnoli S et al (2021) Matching Clinical Diagnosis and Amyloid Biomarkers in Alzheimer’s Disease and Frontotemporal Dementia. Journal of Personalized Medicine 11:47. https://doi.org/10.3390/jpm11010047
Jack CR, Bennett DA, Blennow K et al (2016) A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87:539–547. https://doi.org/10.1212/WNL.0000000000002923
Cicognola C, Janelidze S, Hertze J et al (2021) Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimer’s Research & Therapy 13:68. https://doi.org/10.1186/s13195-021-00804-9
Oeckl P, Anderl-Straub S, Arnim CAFV et al (2022) Serum GFAP differentiates Alzheimer’s disease from frontotemporal dementia and predicts MCI-to-dementia conversion. J Neurol Neurosurg Psychiatry 93:659–667. https://doi.org/10.1136/jnnp-2021-328547
Mazzeo S, Bagnoli S, Ingannato A, et al (2023) Plasma neurofilament light chain predicts Alzheimer’s disease in patients with subjective cognitive decline and mild cognitive impairment: a longitudinal study. https://doi.org/10.1101/2023.05.19.23290183
Benedet AL, Leuzy A, Pascoal TA et al (2020) Stage-specific links between plasma neurofilament light and imaging biomarkers of Alzheimer’s disease. Brain 143:3793–3804. https://doi.org/10.1093/brain/awaa342
Olsen M, Aguilar X, Sehlin D et al (2018) Astroglial Responses to Amyloid-Beta Progression in a Mouse Model of Alzheimer’s Disease. Mol Imaging Biol 20:605–614. https://doi.org/10.1007/s11307-017-1153-z
Rodriguez-Vieitez E, Ni R, Gulyás B et al (2015) Astrocytosis precedes amyloid plaque deposition in Alzheimer APPswe transgenic mouse brain: a correlative positron emission tomography and in vitro imaging study. Eur J Nucl Med Mol Imaging 42:1119–1132. https://doi.org/10.1007/s00259-015-3047-0
Kivisäkk P, Carlyle BC, Sweeney T et al (2023) Plasma biomarkers for diagnosis of Alzheimer’s disease and prediction of cognitive decline in individuals with mild cognitive impairment. Front Neurol 14:1069411. https://doi.org/10.3389/fneur.2023.1069411
Silva-Spínola A, Lima M, Leitão MJ et al (2023) Blood biomarkers in mild cognitive impairment patients: Relationship between analytes and progression to Alzheimer disease dementia. Eur J Neurol 30:1565–1573. https://doi.org/10.1111/ene.15762
Parfenov VA, Zakharov VV, Kabaeva AR, Vakhnina NV (2020) Subjective cognitive decline as a predictor of future cognitive decline: a systematic review. Dement Neuropsychol 14:248–257. https://doi.org/10.1590/1980-57642020dn14-030007
Wen C, Hu H, Ou Y-N et al (2021) Risk factors for subjective cognitive decline: the CABLE study. Transl Psychiatry 11:576. https://doi.org/10.1038/s41398-021-01711-1
Mazzeo S, Padiglioni S, Bagnoli S et al (2020) Assessing the effectiveness of subjective cognitive decline plus criteria in predicting the progression to Alzheimer’s disease: an 11-year follow-up study. Eur J Neurol 27:894–899. https://doi.org/10.1111/ene.14167
Giacomucci G, Mazzeo S, Padiglioni S et al (2022) Gender differences in cognitive reserve: implication for subjective cognitive decline in women. Neurol Sci 43:2499–2508. https://doi.org/10.1007/s10072-021-05644-x
Guest FL, Rahmoune H, Guest PC (2020) Early Diagnosis and Targeted Treatment Strategy for Improved Therapeutic Outcomes in Alzheimer’s Disease. In: Guest PC (ed) Reviews on New Drug Targets in Age-Related Disorders. Springer International Publishing, Cham, pp 175–191
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. This project is funded by Tuscany Region—PRedicting the EVolution of SubjectIvE Cognitive Decline to Alzheimer’s Disease With machine learning—PREVIEW—CUP. D18D20001300002.
Author information
Authors and Affiliations
Contributions
Salvatore Mazzeo: conceptualization; ideas; data curation; formal analysis; investigation; methodology; software; writing; original draft preparation. Giulia Giacomucci: investigation; data curation; visualization. Assunta Ingannato: investigation; resources; validation; visualization. Silvia Bagnoli: conceptualization; investigation; resources; validation; visualization. Arianna Cavaliere: investigation; data curation visualization. Valentina Moschini: investigation; data curation; visualization. Juri Balestrini: investigation; data curation; visualization. Carmen Morinelli: investigation; data curation; visualization. Giulia Galdo: investigation; data curation; visualization. Filippo Emiliani: investigation; data curation; visualization. Diletta Piazzesi: investigation; data curation; visualization. Chiara Crucitti: investigation; data curation; visualization. Daniele Frigerio: investigation; data curation; visualization. Cristina Polito: investigation; data curation; visualization. Valentina Berti: resources; investigation. Sonia Padiglioni: conceptualization; investigation; resources; data curation; visualization. Sandro Sorbi: supervision; visualization. Benedetta Nacmias: funding aquisition; supervision; visualization. Valentina Bessi: conceptualization; ideas; data curation; investigation; methodology; funding aquisition; project administration; supervision; visualization.
Corresponding author
Ethics declarations
Ethical approval
Study procedures and data analysis were performed in accordance with the Declaration of Helsinki and with the ethical standards of the Committee on Human Experimentation of our Institute. The study was approved by the local Institutional Review Board (reference 15691oss). All individuals involved in this research gave informed consent to participate and agreed to have details and results of the research about them published.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Salvatore Mazzeo and Assunta Ingannato are contributed equally.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mazzeo, S., Ingannato, A., Giacomucci, G. et al. The role of plasma neurofilament light chain and glial fibrillary acidic protein in subjective cognitive decline and mild cognitive impairment. Neurol Sci 45, 1031–1039 (2024). https://doi.org/10.1007/s10072-023-07065-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07065-4